AI-powered notch ligand design to enhance T-cell based immunotherapies
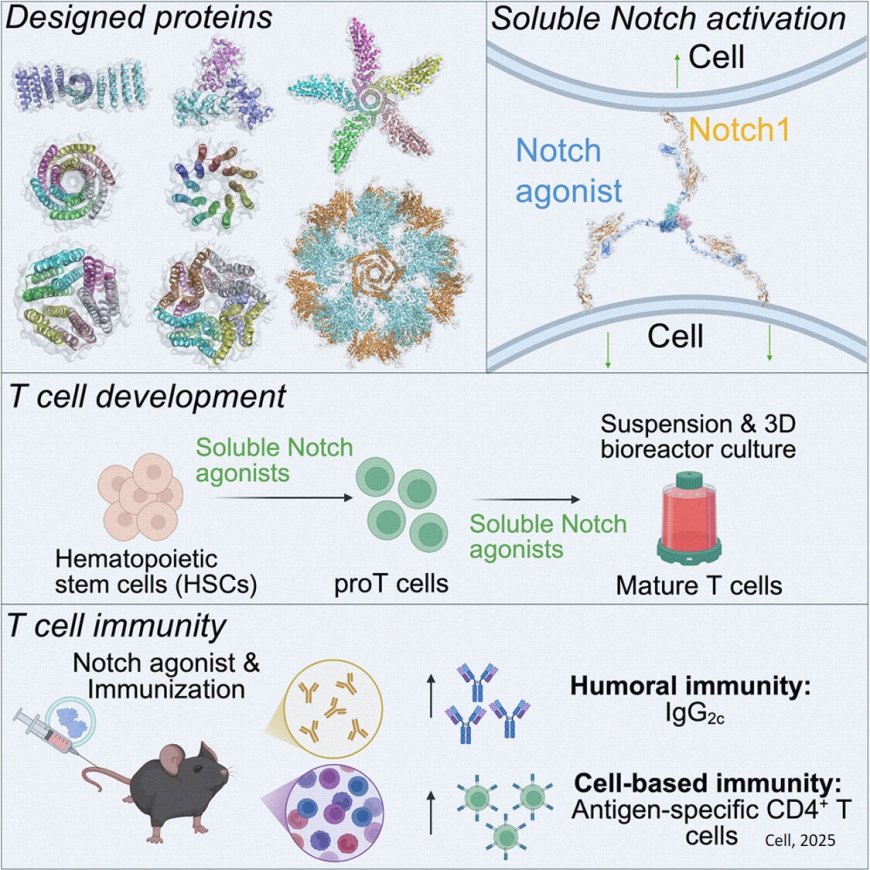
A new paper published in Cell highlights how researchers have leveraged AI-based computational protein design to create a novel synthetic ligand that activates the Notch signaling pathway, a key driver in T-cell development and function. These so-called soluble Notch agonists can be broadly applied to optimize clinical T-cell production and advance immunotherapy development.
Notch signaling is central to many cellular differentiation processes and is essential in transforming human immune cells into T-cells that target viruses and tumors. But activating Notch signaling in the laboratory has posed a challenge. To address this, researchers worked to engineer a soluble Notch agonist to promote T-cell production in liquid suspension culture rather than on a flat “2D” surface.
The team took advantage of recent developments in computational protein design using the Rosetta protein design tool, which enables the design of proteins from scratch. The lab screened a panel of multivalent Notch ligands with different geometries and ligand presentation and tested their capacity for receptor activation. They found that trans-binding configurations best promoted Notch synapse formation, with receptor clustering at the cell-cell junction providing a ‘signaling hub’ for amplified Notch activation.
“AI-driven protein design is a broadly enabling platform technology that we’ve exploited to develop a synthetic molecule that facilitates T-cell manufacture for clinical use and enhances immune responses when delivered in vivo,” says the senior author. “We’re excited that this approach can target T-cells to tumors while also stimulating their cytotoxic functions.”
“Being able to activate Notch signaling opens up lots of opportunities in immunotherapy, vaccine development, and immune cell regeneration,” says the lead scientist for this research. “But what excites me the most is that I’m using this technology to engineer a variety of synthetic protein molecules that bridge T-cells and cancer cells while boosting T-cell killing and neutralizing the immunosuppressive tumor microenvironment. My goal is to make better immunotherapies.”