ALS-causing SOD1 mutants form distinct amyloid fibril structures
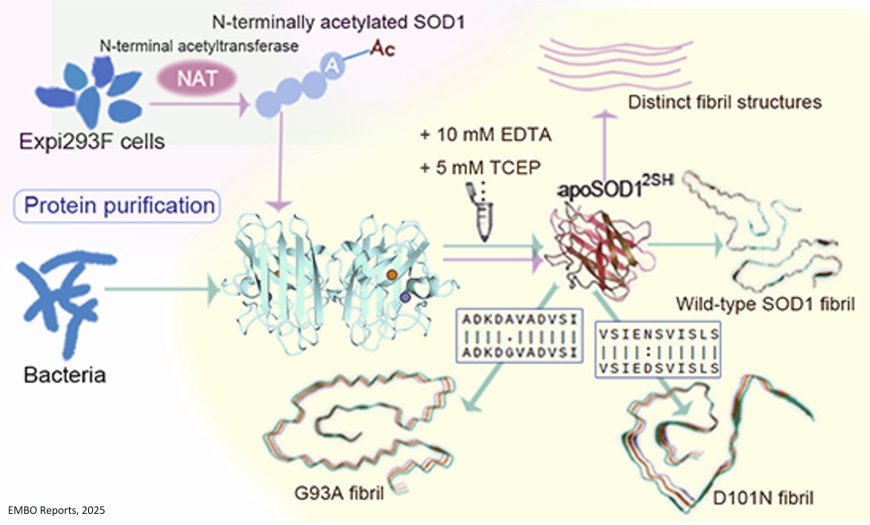
SOD1 mutants haven been linked to amyotrophic lateral sclerosis (ALS) and variants G93A and D101N maintain much of their physiological function, closely resembling that of wild-type.
The researchers in this study unravel cryo-EM structures of E. coli expressed SOD1 mutant (G93A and D101N) fibrils, reveal their distinct assembly and toxicity compared to wild-type SOD1.
The authors show that E. coli expressed SOD1 mutants G93A and D101N form distinct novel fibril structures revealed by cryo-EM.
Fibril seeds from E. coli expressed G93A exhibit markedly enhanced toxicity compared with those from E. coli expressed wild-type SOD1.
N-terminally acetylated SOD1 mutants purified from mammalian cells assemble into structurally divergent fibrils, highlighting the impact of acetylation on fibril polymorphism.
https://www.embopress.org/doi/full/10.1038/s44319-025-00557-8
https://sciencemission.com/amyloid-fibril-structures-formed-by-ALS-causing-SOD1-mutants