Biomolecular condensates implicated in a childhood brain cancer
A study looking at the biophysical properties of an abnormal protein driving cancer cells is giving scientists new therapeutic clues for how to treat ependymoma, the third most common childhood brain tumor. Scientists were studying how the fusion protein ZFTA–RELA, implicated in 95% of ependymomas in the brain cortex, drives disease.
Results of the study demonstrate that disordered regions of the fusion protein cause the formation of droplets within cells called condensates. The researchers revealed that these “membraneless organelles” are essential for ependymoma development. The findings were published in Nature Cell Biology.
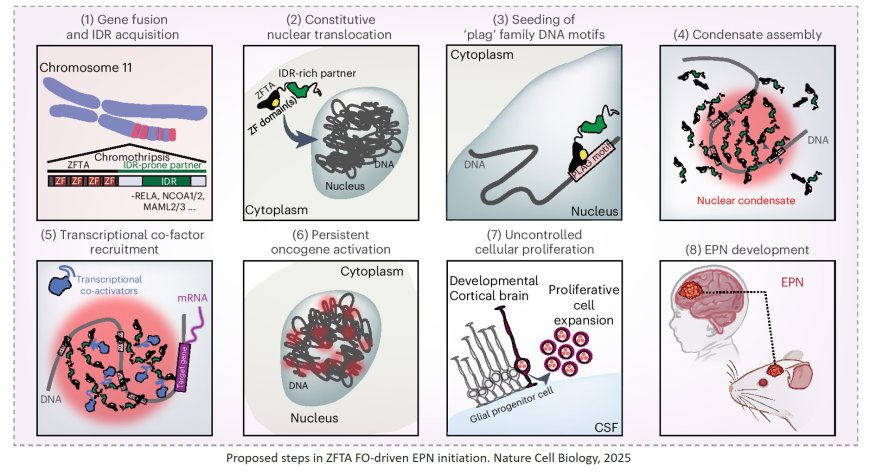
The scientists started their study by examining the different regions of the ZFTA–RELA fusion protein. They found that the ZFTA portion, which binds DNA directly, was required for cancer development. The RELA portion contains a highly disordered region, meaning it lacks a rigid structure and can move flexibly, and was necessary for condensate formation. Condensates are a way cells organize molecules responsible for carrying out various tasks.
When the disordered RELA region was absent, condensates did not form, and ependymoma did not develop in mice. However, when the scientists swapped the RELA portion with other, unrelated disordered protein regions, the novel fusions still formed condensates, driving the oncogene expression that led to brain tumor development.
“Our findings strengthen the view that condensate formation should be considered as a driving mechanism for oncogenic fusion proteins in general,” said the other co-corresponding. “Especially for those that alter chromatin biology.”
In ependymoma, the condensates assemble the molecules responsible for gene expression. Inside the condensates, the ZFTA portion of the fusion protein guides the complex to the DNA encoding certain oncogenes. The researchers showed that this abnormal process is necessary for ependymoma formation, suggesting it could be disrupted for therapeutic effect.
“Fusion proteins such as ZFTA–RELA are challenging drug targets, but our findings provide an indirect approach,” the author added. “Instead of focusing on this fusion protein, we can now start identifying its interacting partners within condensates, examining which are essential for tumor formation and targeting those.”
While the work was done in ependymoma, other cancers driven by other fusion proteins may have a similar vulnerability.
“We discovered a novel mechanism for assembling molecules that underlies the formation of a deadly brain tumor,” the author said. “By understanding these aberrant condensates, we may have found a new place to look for therapeutic interventions for cancers driven by fusion oncoproteins.”