Role of brain’s immune system in social withdrawal during sickness
“I just can’t make it tonight. You have fun without me.” Across much of the animal kingdom, when infection strikes, social contact shuts down. A new study details how the immune and central nervous systems implement this sickness behavior.
It makes perfect sense that when we’re battling an infection, we lose our desire to be around others. That protects them from getting sick and lets us get much needed rest. What hasn’t been as clear is how this behavior change happens.
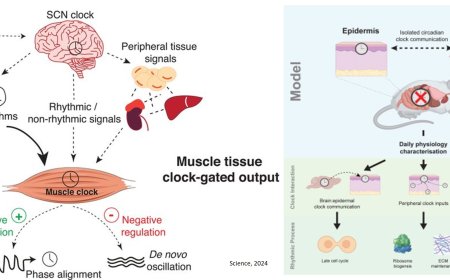
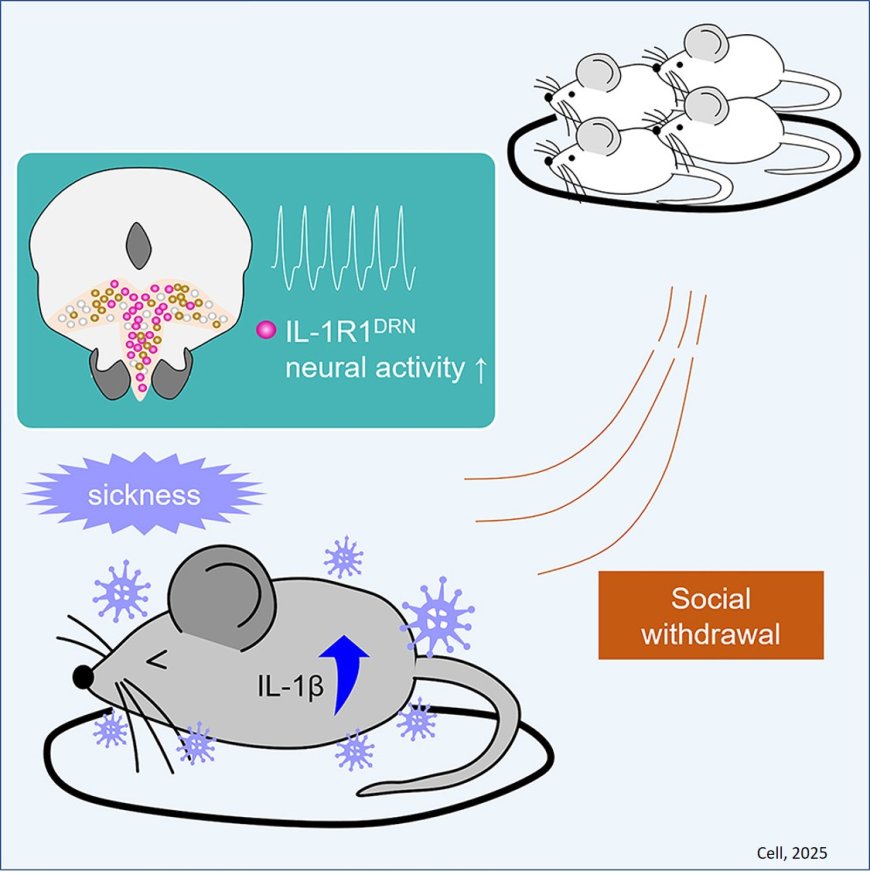
In the research published in Cell, scientists used multiple methods to demonstrate causally that when the immune system cytokine interleukin-1 beta (IL-1β) reaches the IL-1 receptor 1 (IL-1R1) on neurons in a brain region called the dorsal raphe nucleus, that activates connections with the intermediate lateral septum to shut down social behavior.
“Our findings show that social isolation following immune challenge is self-imposed and driven by an active neural process, rather than a secondary consequence of physiological symptoms of sickness, such as lethargy,” said study co-senior author.
The authors have identified other cytokines that affect social behavior by latching on to their receptors in the brain, so in this study their team hypothesized that the same kind of dynamic might cause social withdrawal during infection. But which cytokine? And what brain circuits might be affected?
To get started, the researchers injected 21 different cytokines into the brains of mice, one by one, to see if any triggered social withdrawal the same way that giving mice LPS (a standard way of simulating infection) did. Only IL-1β injection fully recapitulated the same social withdrawal behavior as LPS. That said, IL-1β also made the mice more sluggish.
IL-1β affects cells when it hooks up with the IL-1R1, so the team next went looking across the brain for where the receptor is expressed. They identified several regions and examined individual neurons in each. The dorsal raphe nucleus (DRN) stood out among regions, both because it is known to modulate social behavior and because it is situated next to the cerebral aqueduct, which would give it plenty of exposure to incoming cytokines in cerebrospinal fluid. The experiments identified populations of DRN neurons that express the IL-1R1, including many involved in making the crucial neuromodulatory chemical serotonin.
From there, the team demonstrated that IL-1β activates those neurons, and that activating the neurons promotes social withdrawal. Moreover, they showed that inhibiting that neural activity prevented social withdrawal in mice treated with IL-1β, and they showed that shutting down the IL-1R1 in the DRN neurons also prevented social withdrawal behavior after IL-1β injection or LPS exposure. Notably, these experiments did not change the lethargy that followed IL-1β or LPS, helping to demonstrate that social withdrawal and lethargy occur through different means.
“Our findings implicate IL-1β as a primary effector driving social withdrawal during systemic immune activation,” the researchers wrote in Cell.
With the DRN identified as the site where neurons receiving IL-1β drove social withdrawal, the next question was what circuit they effected that behavior change through. The team traced where the neurons make their circuit projections and found several regions that have a known role in social behavior. Using optogenetics, a technology that engineers cells to become controllable with flashes of light, the scientists were able to activate the DRN neurons’ connections with each downstream region. Only activating the DRN’s connections with the intermediate lateral septum caused the social withdrawal behaviors seen with IL-1β injection or LPS exposure.
In a final test, they replicated their results by exposing some mice to salmonella.
“Collectively, these results reveal a role for IL-1R1-expressing DRN neurons in mediating social withdrawal in response to IL-1β during systemic immune challenge,” the researchers wrote.
Though the study revealed the cytokine, neurons and circuit responsible for social withdrawal in mice in detail and with demonstrations of causality, the results still inspire new questions. One is whether IL-1R1 neurons affect other sickness behaviors. Another is whether serotonin has a role in social withdrawal or other sickness behaviors.