CRISPR editing in nondividing human cells (neurons)
The gene editing tool known as CRISPR-Cas9 is changing what’s possible for treating a wide range of diseases caused by genetic mutations. But so far, attempts to use the technology to address brain-based genetic disorders have proved challenging in the lab.
Scientists have now discovered why. In a study published in Nature Communications, researchers have shown that neurons and other nondividing cells respond differently to CRISPR-Cas9 gene editing than dividing cells.
“Our findings could have a major impact on how gene editing therapies are designed,” says the Senior Investigator. “If we want to ensure genome edits result in the right outcomes, we need to understand and control how the cell’s DNA is repaired after we cut it. Those DNA repair mechanisms are particularly understudied in nondividing cells.”
Gene editing uses a protein called Cas9 as molecular scissors to cut targeted DNA and change its sequence. In the new study, the scientists discovered that gene editing has dramatically different outcomes in neurons than in the cells used in most previous studies.
“Most people studying CRISPR have focused on dividing cells, but it turns out the rules of genome editing are different in nondividing cells like neurons,” says the first author of the study. “This is because different cell types repair DNA damage, such as the cuts made by Cas9, in completely distinct ways.”
The authors reasoned that the unique DNA repair toolkit of neurons might change how they react to the cuts of molecular scissors like Cas9.
To investigate this, the researchers first needed a way to deliver controlled amounts of CRISPR-Cas9 molecules into neurons. So they teamed up with Gladstone Senior, who received a Nobel Prize for the discovery of the CRISPR gene editing technology.
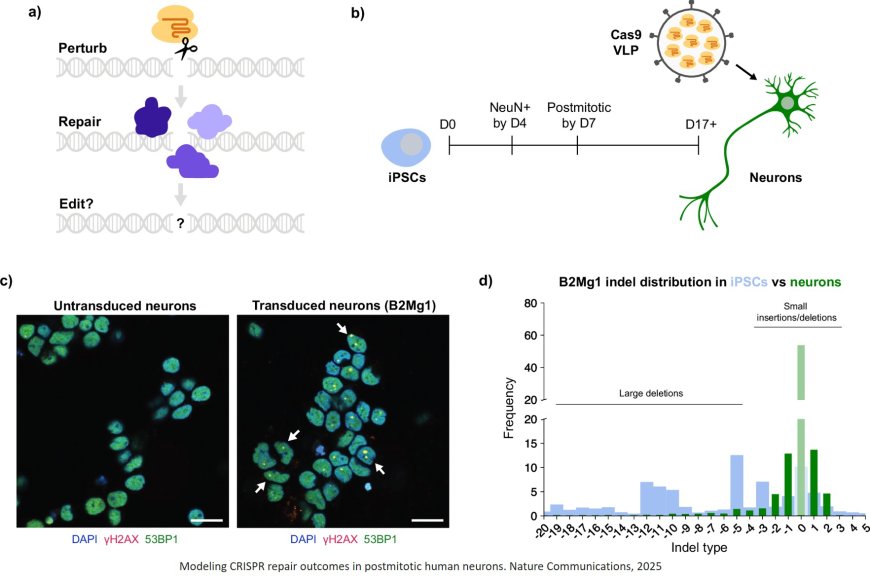
The team generated neurons from induced pluripotent stem cells (iPS cells). Typically derived from skin or blood samples from human donors, iPS cells can be converted into nearly any desired cell type, including neurons. This allowed the team to study dividing and nondividing human cells that shared the exact same genetic code; the key difference is that iPS cells divide and neurons don’t.
Despite delivering the same dose of Cas9 and cutting the same sequence of DNA, the researchers observed wildly different results between the two cell types.
They found Cas9 persists much longer in neurons; while Cas9 editing continued for only a few days in dividing iPS cells, it continued for as long as a month in neurons.
“This could be an important safety consideration,” the author says. “If Cas9 hangs around longer, it has more chances to do its job and make on-target edits, which we want. But it also has more chances to make off-target edits, which we don’t want. We will have to factor this in when designing therapies.”
Once those Cas9 cuts were fully resolved, the repaired DNA sequences also looked dramatically different in neurons. Compared with dividing cells, the spectrum of all possible editing outcomes in neurons was limited to just a few types of changes.
“Imagine if someone slashed the Mona Lisa with a razor,” the author says. “Would you rather have many different people trying to put it back together all at once—one with duct tape, one with superglue, one with a stapler? Or, would you rather have one dedicated expert who repairs it the same way every time?”
Since neurons have fewer options available, the repair outcome can be more predictable and precise.
Perhaps most surprisingly, the team found that the edited neurons activated certain DNA repair genes that were previously thought to be inaccessible to nondividing cells. By investigating these repair genes, the scientists uncovered new ways to direct neurons toward more desirable gene editing outcomes.
“We also showed that these new strategies can be used in other important nondividing cells, including heart muscle cells,” the author says. “Together, these findings will give us and other scientists better control over the safety and efficacy of gene editing.”
While the researchers are still trying to figure out why neurons turn on unexpected repair genes, they have developed novel ways to control them. The team designed lipid nanoparticles to co-deliver their gene editing tools alongside specialized molecules that inhibit these repair genes of interest in neurons.
Using these all-in-one particles, the researchers could make a precise cut in the DNA while simultaneously controlling how that cut is repaired.
“You can think of it almost like doing surgery on the genome,” the first author says. “Before, we could make an incision but we couldn’t control how the DNA gets stitched up afterward. With this work, we’re starting to develop the repair tools required to ensure the right editing outcome.”
Importantly, the platform developed by this study can be directly applied to other gene editing proteins, other cell types, and many different diseases.
“Our ultimate goal is to precisely control the gene editing process to deliver life-changing therapies,” the senior author says. “And now, we have important new tools to make sure we get this right.”