‘Creeping fat’ can worsen Crohn’s disease
Fat is more complicated than we thought. Once considered just a bag of calories, scientists now know that our fat — aka adipose tissue — doesn’t just squirrel away energy; it also sends and receives hormonal, nervous system and immune signals.
Now, a study led by researchers publishing in Cell, shows fat playing another role. A type of abnormal adipose tissue known as “creeping fat” triggers debilitating scarring in the intestines of people with Crohn’s disease, the study found.
This means the stiff, finger-like deposits of creeping fat that encase the intestines of Crohn’s disease patients are not just bystanders; rather, they worsen the disease. The mechanism is surprisingly similar to the way scars form elsewhere, such as on the skin, the researchers said.
Physicians currently lack therapies to keep these scars from forming; the only treatment is surgical excision. The study revealed potential molecular targets for drugs to prevent formation of the scars, known as strictures. Such drugs would be a huge boon to Crohn’s patients.
“These patients really struggle,” said co-senior author. The author treats Crohn’s patients, performing surgery to remove strictures. The patients tell that in addition to feeling physically debilitated, they feel frustrated by being unable to control when or for how long their symptoms will upend their lives. “My heart goes out to them,” the author said. “There’s a lot of suffering here that we think we can alleviate.”
Crohn’s is a severe intestinal disease that commonly begins in the teen or young adult years. It’s marked by cycles of intestinal inflammation, mostly in the far end of the small intestine and the bowel. Patients suffer from abdominal pain, diarrhea, malnutrition, weight loss and fatigue.
Anti-inflammatory drugs sometimes control the problem, but if chronic inflammation takes hold, the intestine can become stiffened and narrowed with scar tissue — a stricture. Strictures, which often form at the valve that joins the small intestine and the bowel, can affect up to 80% of those whose disease isn’t put into remission by anti-inflammatories. Surgery isn’t necessarily a long-term solution.
“The recurrence rate is high, and once you start cutting strictures out, you’re reducing the amount of intestine left,” the author said. “It’s not a journey that patients, or their surgeons or gastroenterologists, are happy with.”
Prior studies noted that patients form strictures in areas of the intestine that have a lot of creeping fat. This type of fat, a hallmark of Crohn’s, forms when healthy fat near the intestines — normally a thin, flexible fat layer called the mesentery — responds to chronic inflammation by becoming abnormally thick and stiff, gradually encasing the diseased intestine.
The author wanted to learn more about the relationship between creeping fat and strictures because, as a surgeon, he kept seeing and thinking about them.
“Most Crohn’s disease research comes from scientists and gastroenterologists, and they have a different experience with this disease — performing endoscopy, prescribing medication, things like that,” the author said. “They don’t get to touch the strictures like surgeons do; we have a very personal experience with this part of the disease.”
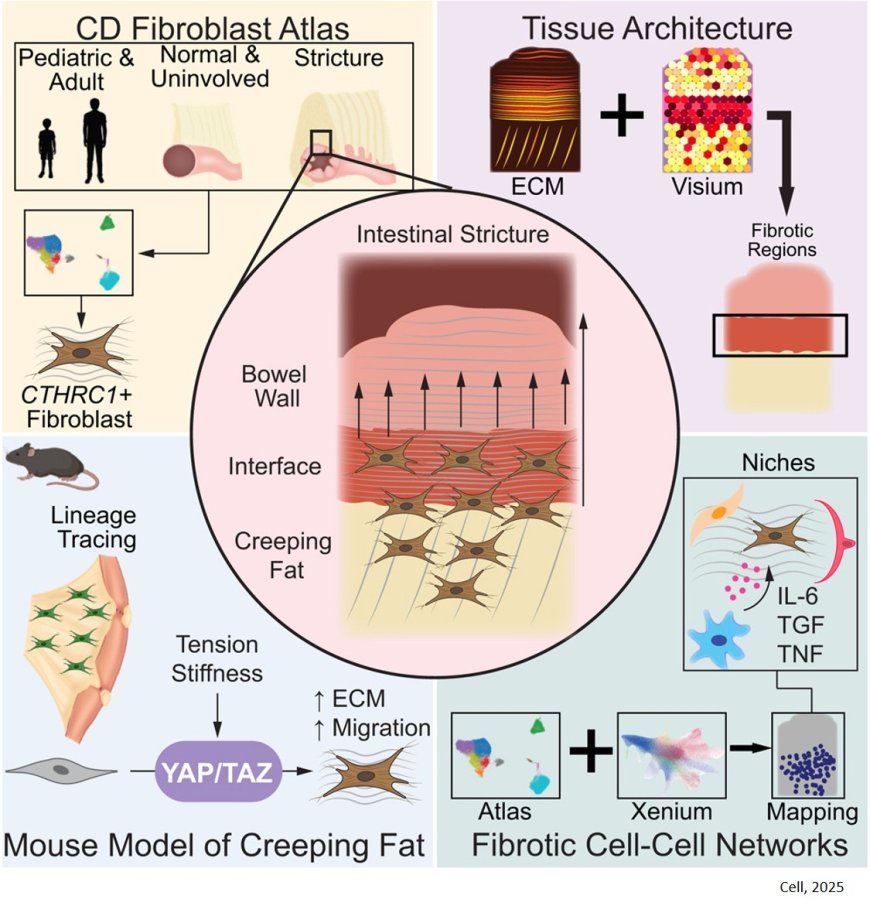
To figure out if creeping fat initiates the formation of strictures, the researchers analyzed tissues taken from humans with and without Crohn’s disease; they also developed a mouse model that accurately replicates the biology of the disease.
Using tissue samples removed from Crohn’s patients during stricture treatment surgery, the team demonstrated that creeping fat contains cells called fibroblasts — capable of generating connective tissue. The genetic pathways switched on in the creeping-fat fibroblasts showed that these cells were primed to detect mechanical strain in the nearby intestine; they were also primed to manufacture more extracellular matrix, the fibrous substance that makes up scar tissue, in response. The researchers looked within the fat-and-bowel samples and found that cells at the interface between the two types of tissue were activating genes involved in making extracellular matrix, i.e. connective tissue, and that the activated fibroblasts clustered in this border region.
After verifying that their mouse model shows features similar to human Crohn’s disease, including strictures, the research team showed that the mice also had fibroblasts at the interface between their fat and intestinal tissue, and that these cells were producing extracellular matrix in response to the intestine being mechanically stretched. Further experiments showed that the combination of inflammation in the intestine plus mechanical tension in intestinal tissue could drive the fibroblasts in nearby fat to participate in stricture formation in the mice.
The interplay between inflammation, mechanical stretch of the intestine and the fat adds to our knowledge of how Crohn’s disease progresses, the scientists said. Scientists had speculated that tension helped trigger strictures because of where the scars tend to form, such as at the valve between the small intestine and the bowel, where the tissue is often stretched.
“The way that many people have thought about inflammatory bowel diseases, including Crohn’s disease, is inside-out, starting with inflammation in the mucosa, the layer closest to where food is digested,” the author said. “Our team now has a different way of thinking about Crohn’s disease; we’re also talking about outside-in. We now think inflammation comes through the entire wall of the bowel and activates the fat, which then launches signals that go back into the bowel and do further damage.”
The lab studies scarring in other tissues, including the skin. That work has led to ongoing clinical trials of a drug that interrupts a signaling pathway known as YAP/TAZ. Signals in the pathway help the fibroblasts form scars by responding to mechanical tension in the tissues.
When the scientists interrupted YAP/TAZ signals in the fat-cell fibroblasts in mice, using the same experimental drug, the mice formed fewer strictures, suggesting a future in which Crohn’s strictures could be prevented in people with a similar medication.
The discoveries help explain why for many Crohn’s patients, anti-inflammatories aren’t sufficient to stop the disease. Inflammation starts the disease process, but at a certain point, Hyun said, the creeping fat becomes biologically primed to have a runaway response to mechanical tension.
“If we don’t deal with that, no matter how good your anti-inflammatory drugs are, patients are still going to form strictures and need surgeries.”