Engineered receptors help the immune system home in on cancer
Most cancer treatments – from chemotherapies to engineered immune cells – have a host of side effects, in large part because they affect healthy cells in the body at the same time as targeting tumor cells. For the same reason, designing new cancer drugs can be challenging due to the molecular similarities between tumor cells and healthy cells.
Now, researchers have designed highly customizable biological sensors which can ensure that engineered cells are only activated in certain environments – such as the vicinity of a tumor. This could yield cancer therapies that are precisely delivered to tumors, making them more effective and giving them fewer side effects than today’s treatments. It also could lead to new, targeted therapies for other diseases.
The breakthrough, described today in Nature, revolves around engineered receptors on the surface of cells that can sense molecules in the surrounding environment and, in response, change the expression of genes inside the cells.
“We can now program a cell to localize to a site of disease and then carry out a very specific set of therapeutic tasks,” said a co-senior author of the new paper. “This is the culmination of more than a decade of work into the molecular details of these receptors and how they can be modified.”
In 2016, a research team developed a new class of sensors, known as synNotch receptors, that could be inserted into cells to reprogram their behavior in response to stimuli. The researchers created sensors on the surface of immune cells, for example, that recognized tumor cells and activated an immune response. However, the receptors had one restriction: they could only recognize molecules that were on the surface of other cells. The system relied on the tight physical interactions between cells.
Since tumor cells often very closely resemble the healthy cells from which they evolved, the proteins found on their surface are also often found on other cells.
“The receptors were limited in scope because they could only be activated by cell surface markers,” explained a co-first author of the new work. “There are many other molecules that tumors produce which might be more useful in identifying the tumor environment.”
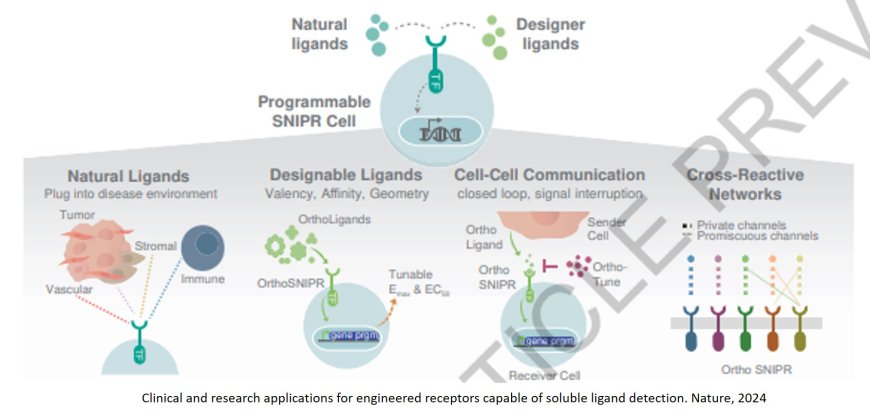
In the years since, the researchers have studied how different elements of the synNotch receptors can be altered to fine-tune their function. That led them to develop new receptors, now called SNIPRs (synthetic intramembrane proteolysis receptors), which can bind to soluble, or free-floating, molecules in the environment around a cell.
The new SNIPRs are engineered to detect any soluble molecule-of-interest, such as an immune signaling molecule. When the molecules bind to corresponding SNIPRs, multiple receptors cluster together and flip to the interior of the cell. There, the receptors directly interact with the DNA inside the cells to alter gene expression. Multiple SNIPRs inserted into one cell could affect different genes – or the same genes in different ways.
“What’s exciting is that we can not only use soluble molecules to flip a genetic switch on, but can customize the SNIPRs so that they turn a genetic program on, turn it off, or dial its activity up and down,” said another co-first author of the paper.
This could mean coaxing a cell to release a drug, activate an immune response, or send signaling molecules to other cells when it is in a particular environment.
A cancer immunotherapy that has been incredibly effective against blood cancers in recent years is chimeric antigen receptor (CAR) T-cell therapy, in which a patient’s own T cells are re-engineered to recognize and attack their cancer cells. However, the therapy has not been as successful in solid tumors, in part because of the difficulty of finding molecules that are unique to the cancer cells for the T cells to recognize.
To show the potential utility of SNIPRs, the researchers inserted newly engineered SNIPRs into CAR T-cells. The SNIPRs were designed to respond to two soluble immune molecules, TGF-β and VEGF, which are often found in high levels around tumors. Only when these molecules were present did the SNIPRs turn on the CAR T-cells’ tumor-fighting activity.
In isolated culture dishes, the researchers showed that the SNIPR-equipped CAR T-cells were only activated in the presence of TGF-β and VEGF, suggesting that they would not launch an immune response in areas of the body unaffected by cancer.
“This is like two-factor authentication for immunotherapy,” said another co-senior author. “The cells have to be in a particular environment to even have the possibility of launching an immune response, which itself requires recognizing cancer cells.”
Indeed, when tested in mice with human tumors, the cells specifically targeted and attacked the tumors, minimizing damage to healthy tissue. Moreover, the treatment shrank the tumors in mice without causing the side effects usually seen using CAR T-cells, including weight loss and organ damage.
“This is incredibly exciting for cancer therapies, but it also could be useful in things like autoimmune diseases where we want to regulate immune cells in certain environments,” said the author.
The researchers are continuing to work on methods for using the SNIPRs in a variety of cell types, using them to mediate communication between different cell types, and testing them in patients in CAR T-cell clinical trials sponsored by Arsenal Bio.