Histone deacetylase 8 regulates the conversion of sensory Schwann cells into their repair phenotype during injury
Following an injury, such as a traumatic crush injury, the peripheral nervous system is itself often able to effectively regenerate. This capacity of regeneration is mainly attributable to the Schwann cells of the peripheral nervous system. These cells are real quick-change artists that can transform themselves into repair cells when needed. Unfortunately, the regeneration of nerve cells can be inefficient in some cases.
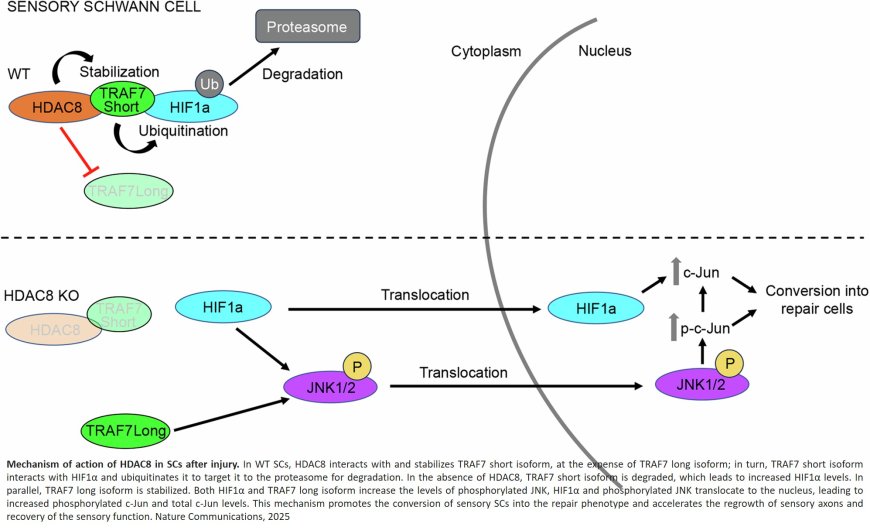
A team of researchers has recently discovered a mechanism that slows down the peripheral nervous system recovery process. "Responsible for this is a protein called histone deacetylase 8, or HDCA8 in short," explained neurobiologist. "This protein is expressed in Schwann cells. If we remove HDCA8, regeneration occurs more rapidly." Interestingly, HDAC8 is specifically produced in Schwann cells surrounding sensory neurons that transmit information on sensations, such as touch, temperature, and pain.
The peripheral nervous system largely owes its ability to regenerate to the high level of plasticity of Schwann cells. Schwann cells, which are also known as neurolemmocytes, provide the myelin sheath that protects the axons of nerve cells. Schwann cells become active immediately after an injury and transform into repair cells that release proteins called neurotrophins. As a result, damaged axons – the long slender projections of nerve cells – can regrow and be guided back to their former targets. Once they reach their targets, Schwann cells remyelinate the regenerated axons, leading to successful functional recovery. This process tends to be particularly efficient in young individuals.
Under certain circumstances, however, where there is a large gap between damaged axons and their intended targets or in older people, reinnervation can partially or completely fail. "It is thus important for us to understand the mechanisms that underlie the plasticity of Schwann cells and determine their function," added the author. The team has now discovered that HDAC8 counteracts the conversion of Schwann cells into their repair phenotype. This transformation process is to some extent triggered by the interruption of oxygen supply that occurs automatically in the case of damage to the peripheral nervous system. "If we get rid of the inhibitor, namely HDAC8, sensory axons regrow faster and sensory function is restored earlier. In fact, the whole process becomes much more efficient," emphasized the author.
The researchers were surprised to find that HDAC8 actually regulates the process specifically in Schwann cells that interact with sensory axons and thereby controls the regeneration of sensory axons and the recovery of sensory functions. "This was new to us. We knew that, upon injury to the peripheral nervous system, Schwann cells change their identity and turn into their repair phenotype. But it turns out this occurs differently in sensory and motor Schwann cells."
According to the author, these new findings raise many conceptual questions. For instance, why do Schwann cells have such a mechanism? One possible explanation could be related to the lack of oxygen, so-called hypoxia, after an injury. In this case, hypoxia promotes the formation of new blood vessels – and HDAC8 could be a regulator of blood vessel formation. The next step could be a drug that removes the HDAC8 protein, prevents its production, or inhibits its function in this process.
https://www.nature.com/articles/s41467-025-55835-9
https://sciencemission.com/Schwann-cells-into-repair-cells-is-regulated-by-HDAC8