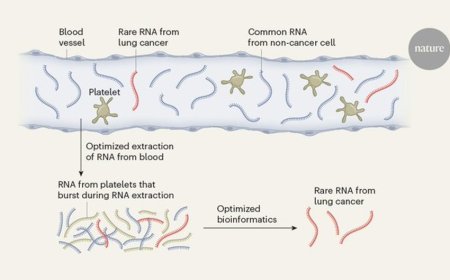
How condensate alter the genomic architecture
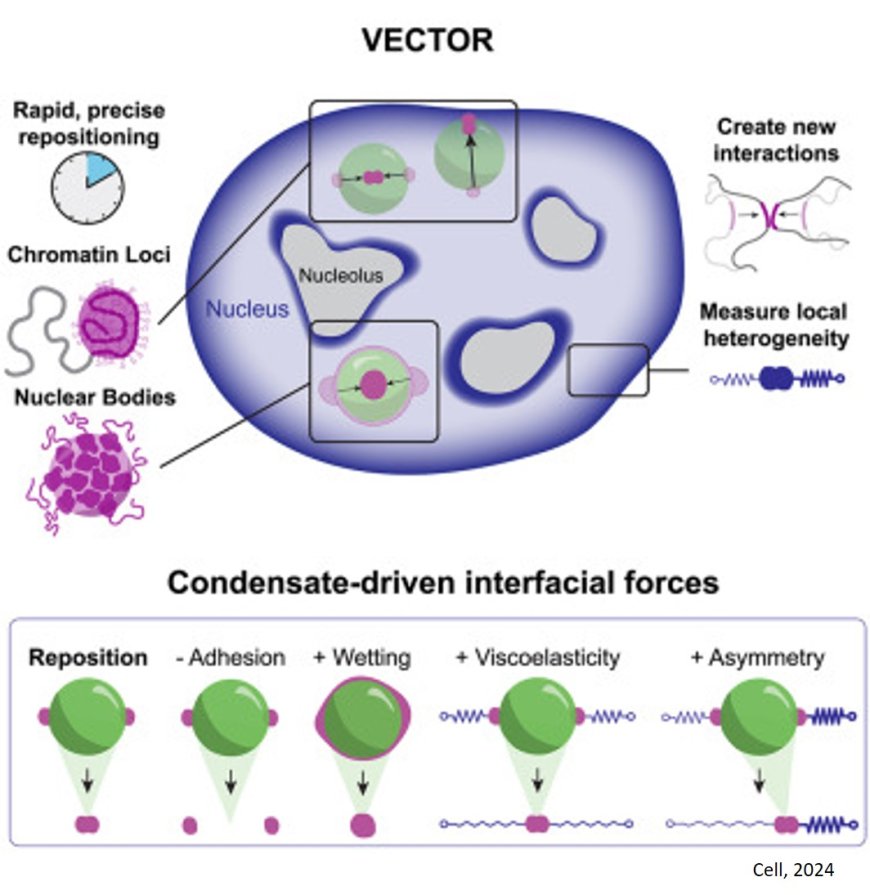
With the flick of a light, researchers have found a way to rearrange life’s basic tapestry, bending DNA strands back on themselves to reveal the material nature of the genome.
Scientists have long debated about the physics of chromosomes — structures at the deepest interior of a cell that are made of long DNA strands tightly coiled around millions of proteins. Do they behave more like a liquid, a solid, or something in between?
Much progress in understanding and treating disease depends on the answer.
A research team has now developed a way to probe chromosomes and quantify their mechanical properties: how much force is required to move parts of a chromosome around and how well it snaps back to its original position. The answer to the material question, according to their findings, is that in some ways the chromosome acts like an elastic material and in other ways it acts like a fluid. By leveraging that insight in exacting detail, the team was able to physically manipulate DNA in new and precisely controlled ways.
They published their findings in the journal Cell.
“What’s happening here is truly incredible,” said the principal investigator of the study. “Basically we’ve turned droplets into little fingers that pluck on the genomic strings within living cells.”
The key to the new method lies in the researchers’ ability to generate tiny liquid-like droplets within a cell’s nucleus. The droplets form like oil in water and grow larger when exposed to a specific wavelength of blue light. Because the droplets are initiated at a programmable protein — a modified version of the protein used in the gene-editing tool known as CRISPR — they can also attach the droplet to DNA in precise locations, targeting genes of interest.
With their ability to control this process using light, the team found a way to grow two droplets stuck to different sequences, merge the two droplets together, and finally shrink the resulting droplet, pulling the genes together as the droplet recedes. The entire process takes about 10 minutes.
Using condensates (green), the researchers pulled two sections of a DNA strand together, enabling them to touch.
Physically repositioning DNA in this way represents a completely new direction for engineering cells to improve health and could lead to new treatments for disease, according to the researchers. For example, they showed that they could pull two distant genes toward each other until the genes touch. Established theory predicts this could lead to greater control over gene expression or gene regulation — life’s most fundamental processes.
A DNA molecule is structured like a long double strand. In living cells, this long strand is wrapped around specialized proteins to form a material called chromatin, which in turn coils on itself to form the structures we know as chromosomes. If uncoiled and stretched end-to-end, all of a person’s chromosomes would measure about six-and-a-half feet long. Human cells must fit 23 pairs of these chromosomes, collectively called the genome, into each cell’s nucleus. Hence the need for tight coiling.
Since DNA is both a carrier of information and a physical molecule, the cell needs to unfurl the tightly coiled parts of the DNA to copy its information and make proteins. The areas along the genome that are more likely to be expressed are less rigid physically and easier to open up. The areas that are silenced are physically more coiled and compact and therefore harder for the cell to open up and read. Like an instruction manual that opens more easily to some pages than others.
The research team turned to blobs of liquid known as condensates to do the work of bending the DNA strands and moving them around.
While some cellular components known to science are like soap bubbles, with a distinct membrane keeping the insides separated from the outside, condensates are liquid-like droplets that fuse together more like raindrops, with no membrane holding them together. After forming and carrying out a cellular function, they can break apart and disperse again.
To study chromatin they utilized an additional component that attaches the condensate to specific locations on the DNA strands and directs their movement quickly and precisely via surface tension-mediated forces also known as capillary forces, which the researchers had suggested could be ubiquitous in living cells. Previously, moving DNA like this relied on random interactions over a period of hours or even days.
“We haven’t been able to have this precise control over nuclear organization on such quick timescales before,” said the author.
Now that they can move the strands around in this controlled way, they can start to look at whether the genes in their new positions are expressed differently. This is potentially important for furthering our understanding of the physical mechanisms and material science of gene expression.
The author said that scientists have looked at the stiffness of the nucleus by poking at it from the outside, and taking a measurement of the whole nucleus. Scientists can also look at one gene and see if it is turned on or off. But the space in between is not well understood.
“We can use this technology to build a map of what’s going on in there and better understand when things are disorganized like in cancer,” said the author.
This new tool is poised to help researchers understand gene expression better, but it is not intended to edit the DNA. “Our tool does not actually cleave the DNA sequences like CRISPR does,” said the author.
“CRISPR is really good for diseases that are related to the need to cut and actually change the DNA sequence,” said the author. This technology could work for a different class of diseases, especially those related to protein imbalances such as cancer.
“If we can control the amount of expression by repositioning the gene,” said the author, “there is a potential future for something like our tool.”