How male hormones regulate skeletal muscle function
Male hormones (androgens), as their name implies, have an important role in promoting the formation of male sexual characteristics (secondary sexual characteristics). In addition, androgens have anabolic effects, as indicated by its alias, anabolic steroid, and administration of androgens is known to cause hypertrophy of skeletal muscle.
Furthermore, androgens regulate gene expression in tissues throughout the body, including skeletal muscle, by binding to a receptor called the androgen receptor (AR). However, it has remained unclear which cells in skeletal muscle are targeted by androgen and by what mechanism of action it regulates skeletal muscle mass.
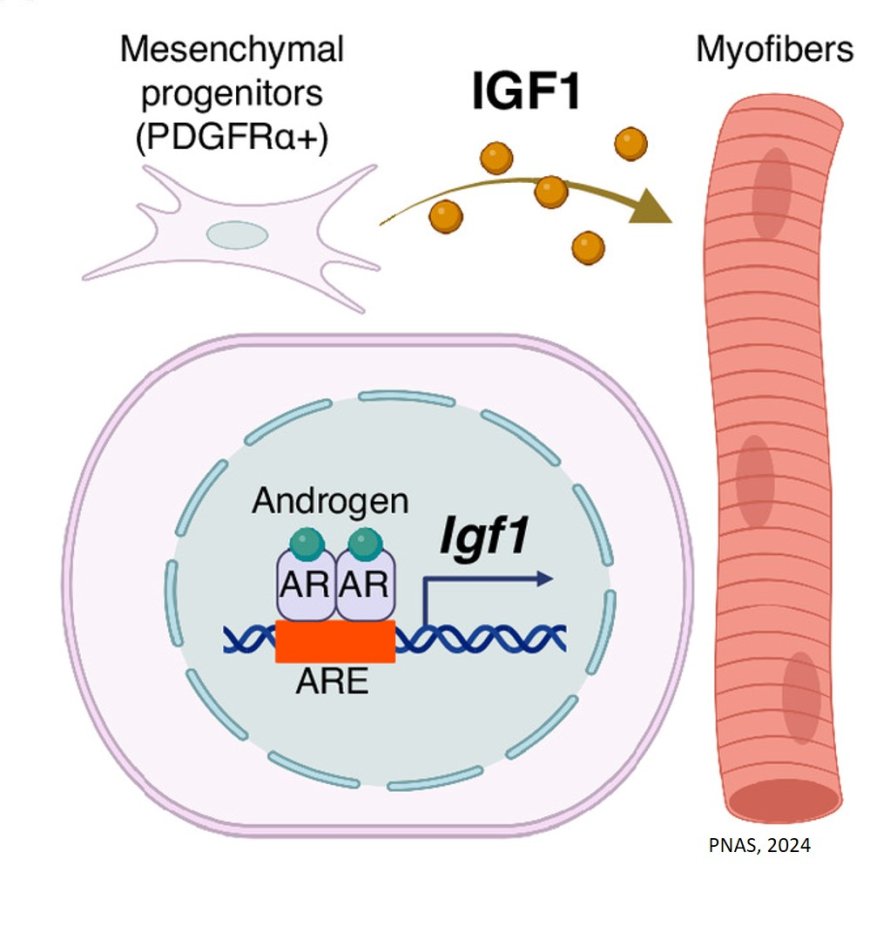
In this study, we focused on mesenchymal progenitors, which are important for skeletal muscle maintenance, to explore how androgens/AR regulate skeletal muscle mass.
The research group first discovered that AR was expressed in mesenchymal progenitors of skeletal muscle by immunofluorescence staining. They then generated mice that lack AR specifically in mesenchymal progenitors (mutant mice) and observed their skeletal muscles. As a result, the mutant mice showed reduced body weight and reduced skeletal muscle weight in the hindlimbs compared to control mice. Furthermore, when the weight of perineal skeletal muscle, known to be sensitive to androgens, was measured, the weight was significantly reduced in 14-week-old, 6-month-old, and 28-month-old mice.
To further explore the cause of this reduction, mesenchymal progenitors were harvested from the perineal skeletal muscle of control and mutant mice and RNA sequencing was performed. The results showed that expression of insulin-like growth factor (Igf1), a protein that regulates skeletal muscle mass, was decreased in the mutant mice.
To further explore the cause of this change in gene expression, mesenchymal progenitors were harvested from hindlimb skeletal muscle of control and mutant mice and CUT&RUN was performed. The results revealed that AR binds to androgen-responsive sequences (ARE) upstream of the Igf1 gene and regulates its expression.
Finally, to confirm whether the reduction of IGF1 due to AR deficiency in mesenchymal progenitors is directly responsible for the reduction of perineal skeletal muscle mass, IGF1 was injected into the perineal skeletal muscle of mutant mice. As a result, the perineal skeletal muscle mass of mutant mice injected with IGF1 was not reduced compared to that of mutant mice injected with saline.
These results indicate that androgens regulate skeletal muscle mass by regulating IGF1 expression via AR expressed in mesenchymal progenitors of skeletal muscle (Figure3). This finding suggests that an appropriate combination of androgens and IGF1 may lead to the development of a new treatment for sarcopenia, an age-related loss of skeletal muscle weight.