Impaired chaperonin function may cause brain malformations and seizures
Most people who visit a doctor when they feel unwell seek a diagnosis and a treatment plan. But for some 30 million Americans with rare diseases, their symptoms don’t match well-known disease patterns, sending families on diagnostic odysseys that can last years or even lifetimes.
But a cross-disciplinary team of researchers and physicians from around the world has solved the mystery of a child with a rare genetic illness that did not fit any known disease. The team found a link between the child’s neurological symptoms and a genetic change that affects how proteins are properly folded within cells, providing the parents with a molecular diagnosis and identifying an entirely new type of genetic disorder.
The results, published in the journal Science, have potential to help find new therapies for rare brain malformations.
“Many patients with severe, rare genetic disease remain undiagnosed despite extensive medical evaluation,” said a co-corresponding author on the study. “Our study has helped a family better understand their child’s illness, preventing further unnecessary clinical evaluations and tests. The findings also have made it possible to identify 22 additional patients with the same or overlapping neurological symptoms and genetic changes that affect protein folding, paving the way for even more diagnoses and, ultimately, potential treatments.”
According to the author, about 10% of patients with suspected genetic disorders have a variant in a gene that has not yet been linked to a disease. His career has been focused on solving such medical mysteries.
The authors used tiny roundworms called C. elegans to assess whether specific genetic changes found in undiagnosed patients are responsible for their symptoms.
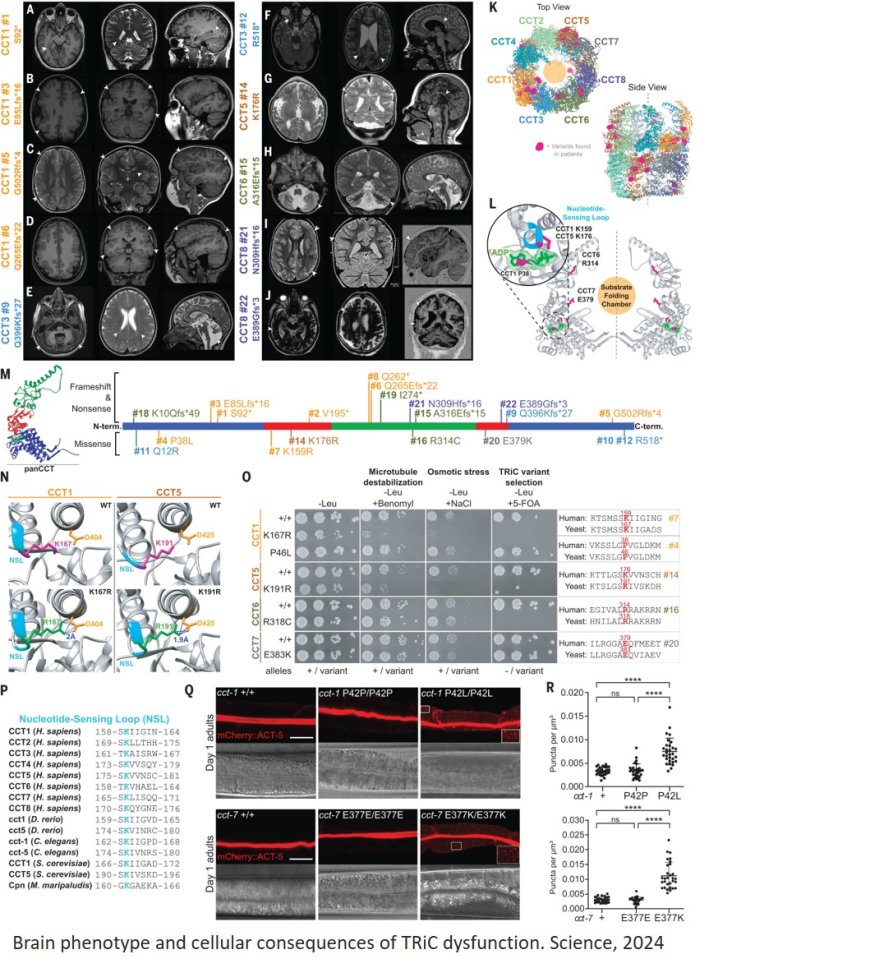
For this study, they teamed up with researchers and doctors from more than a dozen institutions to identify the cause of a cluster of clinical findings in a boy from Germany, and other similar cases. The German patient had an intellectual disability, low muscle tone and a small brain with abnormal structures. Doctors also found changes to the CCT3 gene, so the team set out to determine if it could be the cause of the patient’s condition.
C. elegans has counterparts to about 50% of human genes, including the CCT3 gene, which is known as cct-3 in roundworms. The co-first author, found that C. elegans with the patient’s genetic variant moved slower than roundworms with a healthy copy of the gene did, revealing that the genetic change can affect mobility and the nervous system.
The affected CCT3 protein is part of the large TRIC/CCT molecular complex whose job is to fold other proteins into their proper shape so they function as they should within cells. The study found that the protein-folding machinery cannot perform without a specific amount of healthy CCT3.
“We knew the child has one good and one bad variant gene copy,” the author said. “Our studies in C. elegans revealed that the genetic change reduces the activity of the normal protein, decreasing the capacity of the protein-folding machinery, and that for both C. elegans cct-3 and human CCT3, having 50% of activity was insufficient for normal biological function.”
The outcome of having reduced protein-folding machinery, they found, was that actin proteins – which help to maintain cell shape and movement –were incorrectly folded and abnormally distributed throughout the cells of C. elegans that carried the patient’s variant.
“An understanding of the impact of the genetic change informs the treatment modality,” the author added, “because the treatment needed to increase the amount of a normal protein differs from the treatment needed when the protein is poisonous or overactive.”
The researchers performed complementary investigations into cct3 variants in zebrafish – which illuminated the effects of the gene on brain development – and in yeast, which clarified its role in protein folding, respectively.
To see if there are other patients out there with this same disorder, researchers mined a freely accessible global database of individuals with intellectual and developmental disabilities. They identified 22 individuals with genetic changes in seven of the eight CCT proteins that form the protein-folding machine. Abnormalities in mobility and actin folding were again seen in roundworms with variants affecting CCT1 and CCT7 proteins, just as the team observed with dysfunctional CCT3. Together, these patients represent a new type of rare genetic disease involving the protein folding machinery.
“This work underscores the importance of using simpler model organisms, like C. elegans, to provide novel insights into human pathobiology,” said the co-author.
“Our findings can inform clinicians, the scientific community, and patients and families all around the world that changes to the genetic message that are needed to make the eight-protein complex cause disease,” added the author. “If next week a patient with brain malformations and neurological symptoms is found to have a variant that affects the protein-folding machine, the patient will receive a diagnosis.”
https://www.science.org/doi/10.1126/science.adp8721
https://sciencemission.com/Brain-malformations-and-seizures-by-impaired-chaperonin-function-of-TRiC