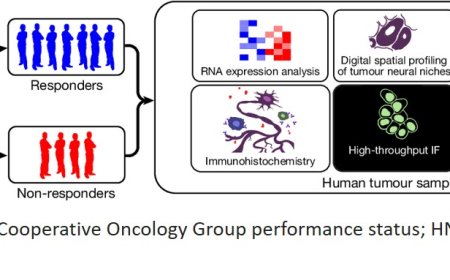
Lysosome-to-mitochondria iron transfer regulate melanoma states
Melanoma, one of the most aggressive skin cancers, often exhibits a remarkable ability to change its phenotype, enabling it to evade therapies and metastasize. While previous studies focused on genetic mutations, new work shifts attention to the cellular machinery that governs iron distribution inside cancer cells. This process is essential for energy production, survival, and the spreading of cancer cells.
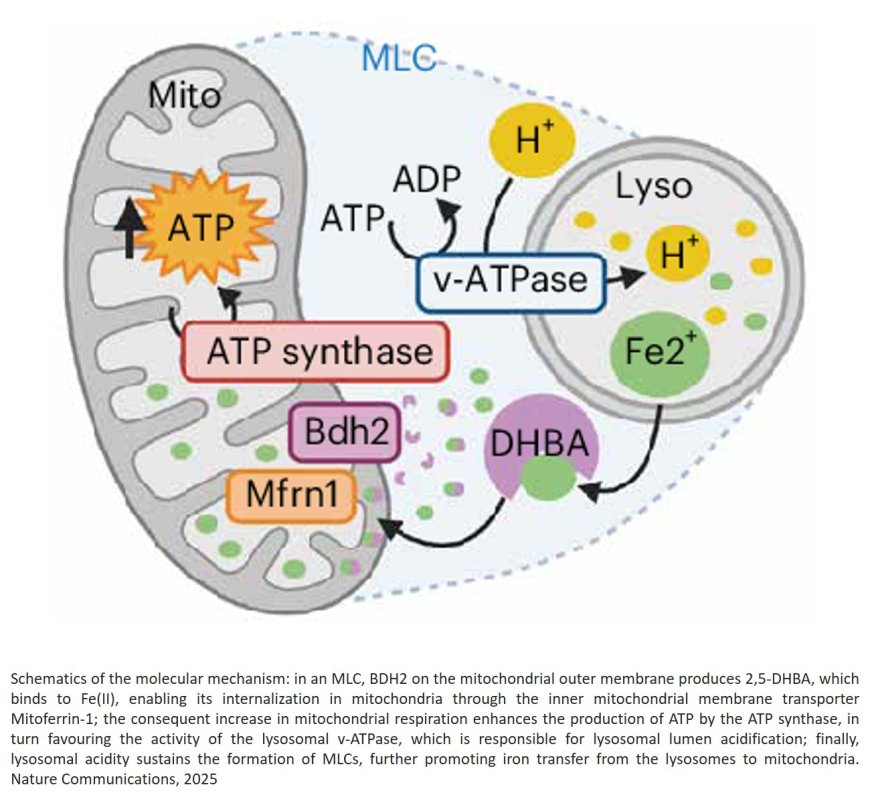
To adapt to their environment, melanoma cells reversibly switch between two major states: the melanocytic (MEL) cell state, which is generally more susceptible to current antimelanoma therapy, and a mesenchymal-like (MES) state with invasive features, which is ultimately responsible for therapy resistance and recurrence. The team discovered that the MES cells harbor disrupted iron transport between the mitochondria and lysosomes — organelles integral to cellular iron storage and utilization- and identified an evolutionarily conserved molecular machinery for the transport of iron between these organelles.
“We found,” says the first author of the study, “that this disrupted iron transport is the result of downregulating an enzyme called BDH2, which produces a molecule that captures iron and transports it into the mitochondria, just like bacteria use it to import iron for their survival and growth. Reducing BDH2 allows iron to accumulate in the lysosomes and to maintain the invasive phenotype. However, there is also a price to pay, as this process exposes MES cells to an iron-induced cell death called ferroptosis.”
Importantly, the scientists demonstrated that restoring the production of BDH2 in MES cells reestablished proper iron trafficking, revitalized mitochondrial activity, and decreased ferroptosis sensitivity when melanoma cells circulate in the unfavorable oxidative environment of the bloodstream.
"Our findings reveal a previously unappreciated layer of metabolic regulation that drives melanoma phenotypic switching and couples organelle iron transfer to the melanoma’s ability to undergo ferroptosis, a form of cell death with the potential to target the population of drug-tolerant cancer cells," says the senior author. "Targeting iron homeostasis and the machinery that maintains organelle crosstalk could offer innovative strategies to prevent tumor progression and overcome resistance."
As researchers continue to untangle the complex interplay between cellular metabolism and tumor behavior, these insights could lead to more effective treatments for melanoma and potentially other cancers exhibiting similar metabolic plasticity.