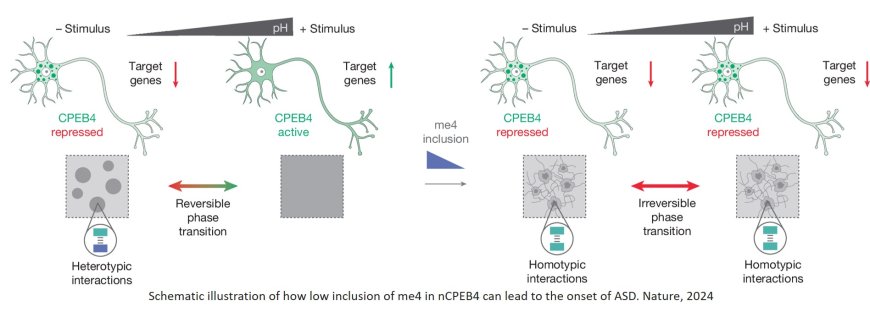
Neuronal microexon mis-splicing promotes CPEB4 aggregation in ASD
Autism is a neurodevelopmental disorder characterised by difficulties in communication and social behaviour. Approximately 20% of cases are linked to a specific genetic mutation, but the origin of the remaining 80%, known as idiopathic autism, remains a mystery.
A team of scientists has identified a molecular mechanism that explains why certain alternations of the neuronal protein CPEB4 are associated with idiopathic autism.
The study is based on previous work published in 2018 that identified CPEB4 as a key protein in the regulation of neuronal proteins related to autism. Back in 2018, the researchers observed that, in individuals with autism, the CPEB4 protein lacked a specific neuronal microexon — a tiny segment of genetic material crucial for protein function in the neurons. The work published in the journal Nature reveals that this small fragment is key for neuronal activity because it preserves the flexibility of CPEB4 to assemble into condensates and disassemble them.
“This study provides new insights into how small modifications in proteins that regulate gene expression can have a significant impact on neuronal development, opening new avenues to explore future therapies,” explains co-corresponding author.
The region of the CPEB4 protein that holds the segment lacks a well-defined three-dimensional structure. Proteins with disordered regions can form condensates, which are like small droplets within the cell where molecules, such as messenger RNAs (mRNAs) that code for other proteins involved in neuronal function, are stored in a silenced state. These condensates can assemble and disassemble in response to cellular signals, enabling dynamic regulation of gene expression.
"In this study, we’ve discovered that this neuronal microexon is crucial for maintaining the stability and dynamics of the condensates formed by CPEB4 in neurons. Without the microexon, the condensates are less dynamic and can form solid aggregates that don’t work correctly," says the other co-corresponding author.
This lack of dynamism prevents the mRNAs stored in these condensates from being released when neurons are stimulated, leading to a decrease in the production of proteins essential for neuronal development and function. Among these mRNA molecules are many of the genes that have previously been linked to autism.
Proper regulation of these genes is essential during brain development. If these CPEB4 condensates do not function correctly due to the absence of the neuronal microexon, it can lead to disruptions of neuronal development, which are manifested as symptoms of autism. The described mechanism also helps to explain the complexity and heterogeneous nature of idiopathic autism, as this spectrum includes multiple manifestations and varying degrees of severity.
"Our results suggest that even small decreases in the percentage of microexon inclusion can have significant effects. This would explain why some individuals without a gene mutation develop idiopathic autism," explain first authors of the study.
The concept proposed in this study of gene regulation in neurons through the formation of condensates may also have implications for ageing. Over time, these condensates lose their plasticity, meaning their capacity to assemble and disassemble, which could impair proper neuronal function and promote the development of neurodegenerative diseases.
One of the promising findings of the study is that microexon 4 appears to work "in trans", which means that it might be possible to introduce this small sequence of amino acids into cells to partially restore CPEB4 function and potentially reverse the symptoms.
"Although we’re still in exploratory stages, this discovery is promising and points to a potential therapeutic approach that could restore CPEB4 function," says the author. The researchers emphasize that this finding still requires extensive experimental testing, such as studies in animal models and overcoming multiple technical barriers.
https://www.nature.com/articles/s41586-024-08289-w
https://sciencemission.com/Mis-splicing-of-a-neuronal-microexon-promotes-CPEB4-aggregation-in-ASD