PLK1 docking motifs and activation mechanisms
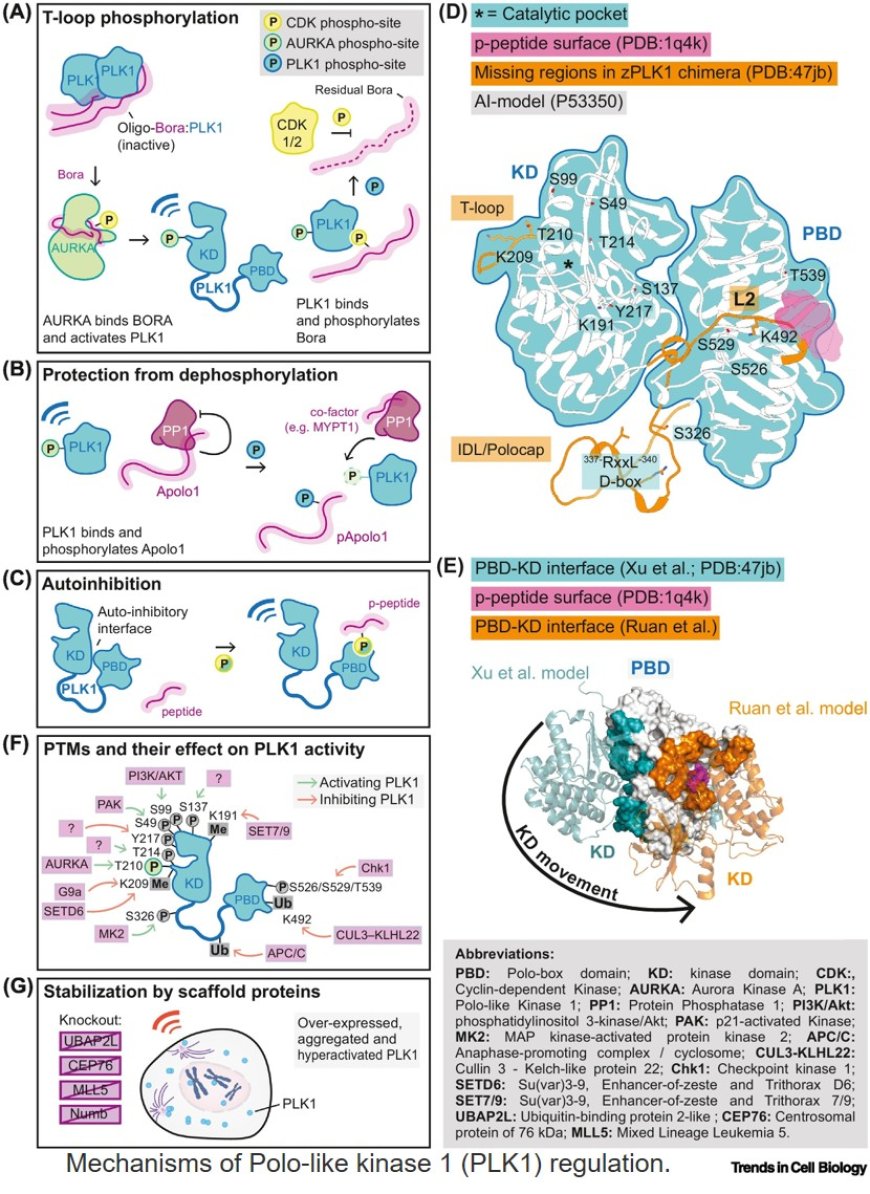
Polo-like kinase 1 (PLK1) activity must be regulated throughout the cell cycle. To do so, the cell adopts several mechanisms, ranging from autoinhibition, localized targeting, and oligomerization to post-translational modifications and PLK1 stabilization via chaperones/scaffold proteins.
T-loop phosphorylation of PLK1 is mainly mediated by the activity of the Aurora ABora complex before mitotic entry, through a CDK1-dependent switch.Bora-PLK1 oligomerization has been proposed to regulate the process in time and space.
PLK1 interacts with docking sites through its Polo-box domain, from which it sequentially phosphorylates additional substrates. Accessory binding sites other than the canonical S-pT-X site are necessary to generate a strong PLK1-docking site.
Recent studies suggest that the Polobox domain of PLK1 acts as a mechanical effector, for instance by relieving an autoinhibitory switch after phosphorylation and binding of a target protein.
https://www.cell.com/trends/cell-biology/fulltext/S0962-8924(25)00156-4