Post translational modification of the same amino acid twice in cells!
Usually, a single amino acid is modified with a single post-translational modification (PTM) at any given time. Recent studies indicate crosstalk between different PTMs, some occurring on the same residue.
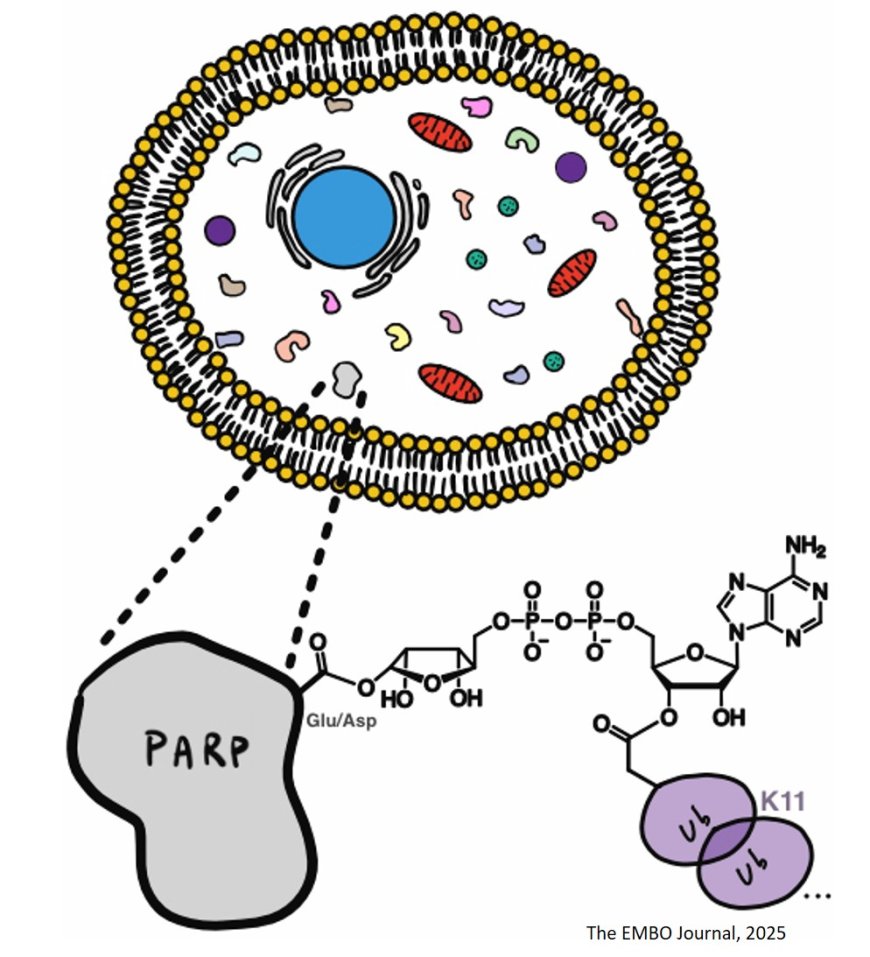
Certain E3 ligases have been found to ubiquitylate hydroxyl groups on free NAD+ and ADP-ribose in vitro, but the in vivo occurrence of this dual post-translational modification has remained uncertain.
This study identifies mono-ADP-ribosyl ubiquitylation (MARUbylation) at the endogenous level in response to IFN-β stimulation, implicating it in innate immune signaling.
MARUbylation occurs in cells, where ubiquitin is ester-linked to mono-ADP-ribosylated Glu/Asp residues on PARP10.
The mono-ADP-ribose ubiquitin ester (MARUbe) is further extended with K11-linked polyubiquitin chains.
The authors show that MARUbylation occurs on multiple interferon-stimulated PARPs such as PARP7 and PARP14, suggesting a common role for MARUbylation in interferon-primed immune responses.
https://www.embopress.org/doi/full/10.1038/s44318-025-00391-7
https://sciencemission.com/Ubiquitination-of-mono-ADP-ribose