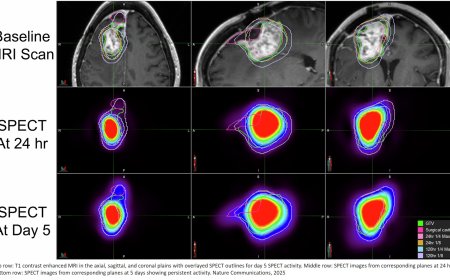
Combination of RNA splicing factor and immunotherapy to treat neuroblastoma
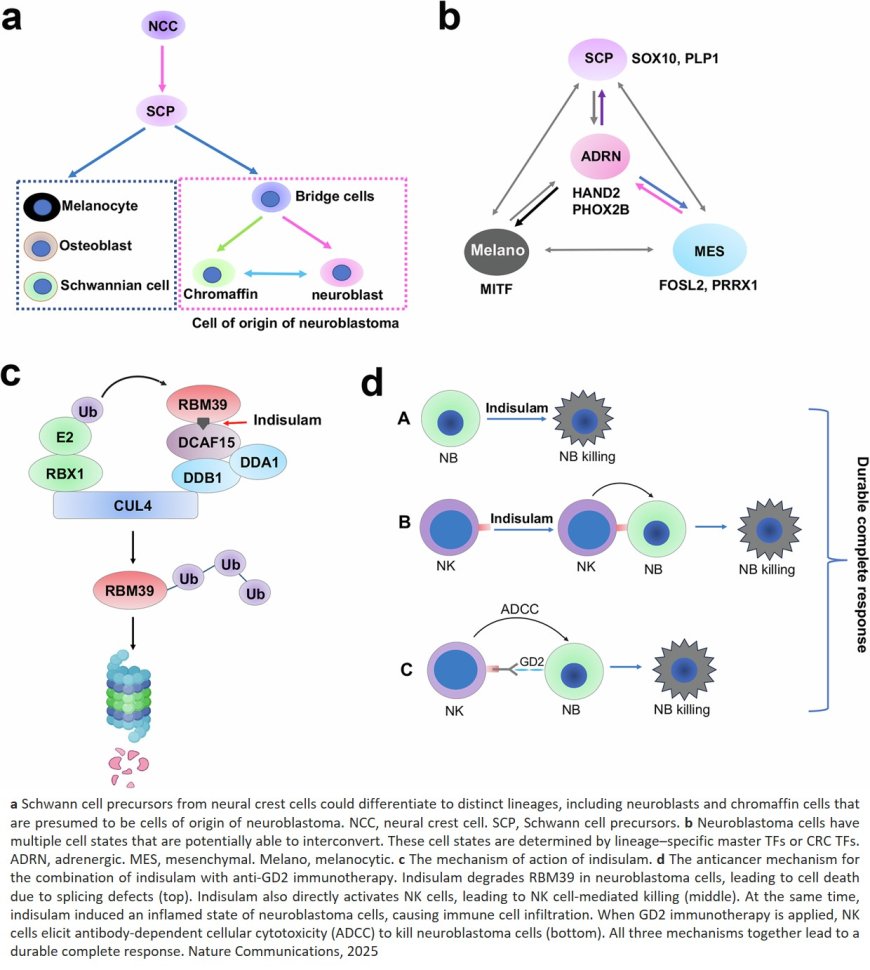
Gene expression governs whether neuroblastoma tumor cells exist in either the adrenergic (differentiated and sensitive to treatment) or mesenchymal (less differentiated and therapy-resistant) cell state. Tumors may switch between states, confounding treatment efforts. A study indicates that the extent of this plasticity is underreported, but there is a way to circumvent this challenge therapeutically.
The researchers found that by combining immunotherapy with the drug indisulam, they could achieve a complete therapeutic response in laboratory models, regardless of cell state. The study, published in Nature Communications, is the first to show how indisulam works and provides a missing piece of the puzzle for how to treat neuroblastoma effectively.
Neuroblastoma is a pediatric cancer that affects nerve tissue and occurs when neural cells fail to develop properly, remaining perpetually locked in an early stage of development. This cell immaturity is intrinsic to tumor cell plasticity. While surgery alone can successfully treat most patients with low-risk neuroblastoma, high-risk patients, who account for almost half of all cases, often need high-dose chemotherapy. When combined with a low number of druggable targets, significant cytotoxic effects for such therapies and a 50% relapse rate, the need for novel neuroblastoma targets is critical.
Corresponding author recently identified the potential of a class of drugs called molecular glues to address this need. “Neuroblastoma cells are highly dependent on a process called RNA splicing,” the author explained, “So by ‘sticking’ an RNA splicing factor, RBM39, to protein-degrading machinery and forcibly degrading it, cell growth can be stopped.” Indisulam is one such molecular glue. While experiments showed high anti-cancer activity, models still consistently relapsed. The researchers worked to understand why, ultimately finding a way to make the drug work more effectively.
The researchers initially encountered confounding results. “We used a genetic model, a patient-derived xenograft mouse model, and a cell line-based model, and all three models showed different RNA sequencing patterns,” the author said. “We couldn’t find common features; every tumor had a unique pattern.”
By projecting these features onto the different stages of cell development, they determined that cells were undergoing unexpected multidirectional switches during development, notably switching from the mesenchymal to adrenergic cell state and vice versa. In some cases, they even acquired additional features, highlighting an unexpected depth to the plasticity of neuroblastoma cells.
“This data helped us understand the extent of heterogeneity in the tumors, and why developing a therapy is challenging; cells will simply switch to another state,” the author explained. “Combining all the drugs to target each sub-cluster is not possible either, due to the toxicity. This is why the indisulam findings, when combined with immunotherapy, are remarkable.”
As part of the cell state switch triggered by indisulam, the researchers observed an innate immune response. They determined that a special type of immune cell called a natural killer cell is recruited to the tumor and that this immune response is at the center of indisulam’s mechanism.
The response was accompanied by an increase in GD2 expression, a protein that resides on the surface of cancer cells and is a key target for immunotherapy. They found that by supplementing indisulam treatment with anti-GD2 immunotherapy, the combination was a one-two knockout punch. “Indisulam can directly activate natural killer cells, and anti-GD2 exerts a mechanism called antibody-dependent cellular cytotoxicity,” the author explained. “It’s two mechanisms that work together, the cellular toxicity and natural killer cells, to eradicate the tumor cell.” The therapeutic approach is being further developed for clinical testing.