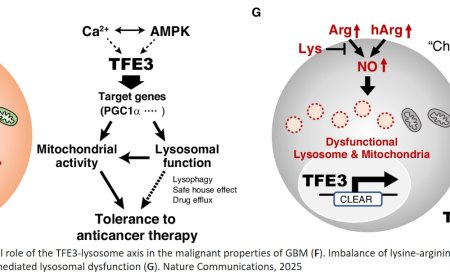
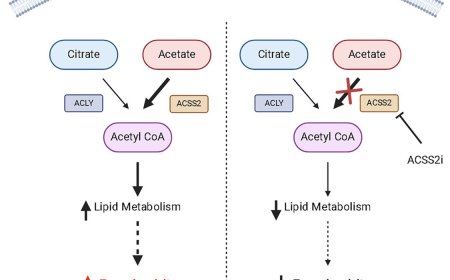
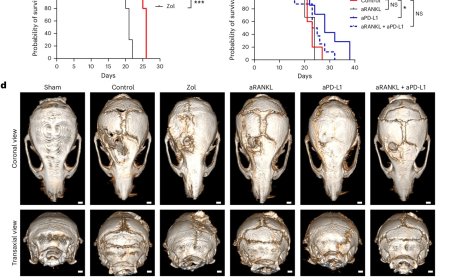
How small cell lung cancer hijacks neuronal synapses
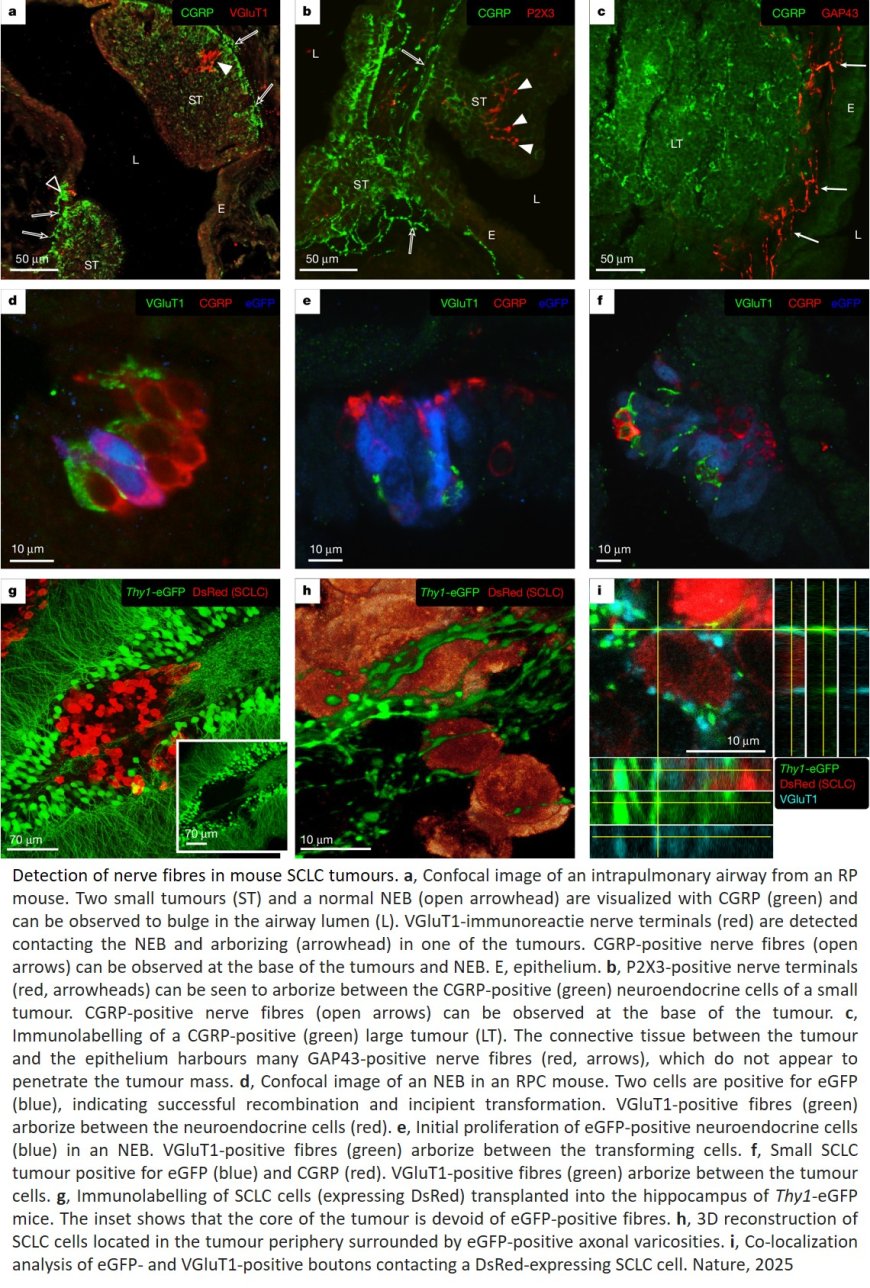
An international research team has shown that lung cancer cells can form functional synapses with neurons, effectively hijacking the body’s neural circuits to grow faster. The finding reveals a startling new dimension of cancer biology and opens promising new avenues for therapies against this disease. The study was published in Nature.
Previously, synapses had only been seen in brain tumors, which arise from the nervous system itself. Finding that a lung cancer can wire itself into neural circuits reveals how deeply a tumor can integrate with the host to survive and thrive. “Our study underscores the alarming extent to which the organism can communicate with and nurture a tumor, supporting its growth as if it were a healthy tissue,” said the study author.
Starting from the analysis of genetic data, the researchers identified a set of genes involved in synaptic formation and went on to visualize the presence of synapses between small cell lung cancer (SCLC) cells and neurons in cell culture and in mouse models.
“I was stunned to see the extent of innervation that SCLC cells manage to co-opt,” said senior and co-corresponding author. “I believe that these findings can become transformative for the development of more efficient therapies to prevent SCLC from metastasizing to the brain.”
The researchers detected neuron-to-cancer synaptic communication through two different neurotransmitters: glutamate and GABA. They further found that SCLC cells proliferated faster in the presence of sensory or cortical neurons. “By forming synaptic connections with different types of neurons, SCLC cells reveal an impressive adaptability to boost their growth,” said one of the senior and co-corresponding authors. “It is tempting to speculate that SCLC cells are not only ‘chatting’ with neurons but are also receiving resources from them to support their growth.”
Crucially, disrupting glutamate signalling led to lower tumor burden and longer survival of the experimental mice, highlighting promising new targets for cancer therapy. “Our observations may open a path forward for the implementation of novel therapeutic interventions in SCLC,” said lead corresponding author. “We have shown that pharmacological disruption of the cancer–neuron crosstalk leads to improved tumor control and that such treatments can be combined with chemotherapy.”
These discoveries suggest several therapeutic possibilities, from repurposing existing neurotransmitter-blocking drugs to developing entirely new approaches targeting cancer–neuron communication. The research team continues to investigate the molecular details of these synaptic connections, work that will be essential for optimizing treatment strategies and identifying the most promising therapeutic combinations.