Co-located myeloid and tumor stem cells drive aggressive brain tumors
A type of aggressive, treatment-resistant brain tumor has a distinct population of immune cells that support its growth, according to new research.
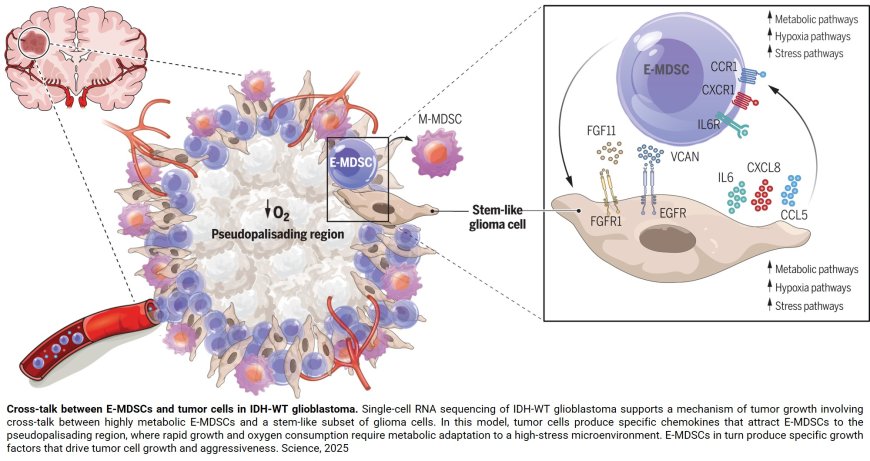
Searching for subtypes of immune cells seen only in the most serious, grade 4 brain tumors, called glioblastomas, and using a recently developed technology called spatial genomics, the researchers found that glioblastoma stem cells were co-localized with a type of immunosuppressive cell called myeloid-derived suppressor cell (MDSC), and that these two cells symbiotically feed off of each other to promote tumor growth and aggressiveness. A description of the work was published in the journal Science.
“Tumor stem cells represent only 5% to 10% of the tumor, but they’re the critical cells that are renewing and generating the rest of the tumor and are essentially responsible for the aggressiveness of the tumor,” says the senior study author. “We found that the myeloid-derived suppressor cells and tumor stem cells literally were in the same place — a region described by pathologists in the 1980s as the pseudopalisading region. There was a very intimate connection.”
To better characterize the cellular components of brain cancer, investigators performed single-cell RNA sequencing on tissue samples from 33 types of brain tumors spanning from low to high grade, finding two populations of MDSCs in IDH-WT glioblastoma. Then, using a technique called spatial transcriptomics to look at patterns of gene expression of over 750,000 immune cells and more than 350,000 tumor and associated cells in these samples, they found MDSCs were co-located with the tumor stem cells.
“Glioblastoma is a highly aggressive brain tumor with remarkable ability to evade the immune system, which has made immune-based therapies largely ineffective to this point,” said first and co-corresponding author. “Our study revealed a distinct subset of immune cells, known as myeloid-derived suppressor cells that promote glioblastoma growth, providing new insights into how the tumor interacts with the immune system. By identifying these cells and their role, we hope to uncover new therapeutic targets and lay the groundwork for more effective treatments.”
In their studies, investigators discovered that the two types of cells were feeding each other in the brain tumors. Tumor stem cells were producing chemical signals called chemokines that attracted the MDSCs, and making growth factors and activation factors for the MDSCs. In turn, the MDSCs were producing growth factors for the tumor cells.
The researchers were able to further ascertain what specific molecules tumor stem cells were producing to attract and activate MDSCs. Two of the key ones identified by the team were IL (interleukin)-6 and IL-8, which play a role in inflammatory responses, and for which MDSCs have receptors.
“IL-8 is one of the major attractants to bring the MDSCs to the tumor, and IL-6 is one of the major activators of the MDSCs,” the author says.
On the flip side, the team found that MDSCs secreted a growth factor called fibroblast growth factor 11 (FGF11) to feed the stem cells, a molecule never before known to be involved in brain or other cancers.
Along the way, the authors found that tumors with a mutation in the IDH1 gene, which are less aggressive, had almost no MDSCs and far fewer cancer stem cells. This led them to look across all brain cancers at the correlation between MDSC infiltration and survival. Using the National Cancer Institute’s Cancer Genome Atlas (TCGA) database of cancer samples, they indeed found that very tight correlation — the fewer cancer stem cells and fewer MDSCs a person had in their tumors, the better they did.
While additional studies are needed to further understand these cellular interactions, the work is exciting in that it suggests additional potential targets to block in treatment of these aggressive brain tumors, the author says. For example, the authors have developed an investigational bispecific antibody that binds to the receptors for IL-6 and IL-8, blocking their signaling.