Mapping the molecular blueprint of blood’s secret messengers
Researchers have unlocked the molecular secrets of microscopic particles circulating in our blood, revealing in a landmark discovery how the body’s cells communicate in ways we’re only beginning to understand.
The research was published in Nature Cell Biology, is the first to chart the complete molecular blueprint of extracellular vesicles (EVs) - nanosized bubbles that act as the body’s invisible messengers.
The senior author said the discovery transformed how scientists viewed EVs.
“By decoding these EV messages, we’re beginning to read the body’s own health reports.”
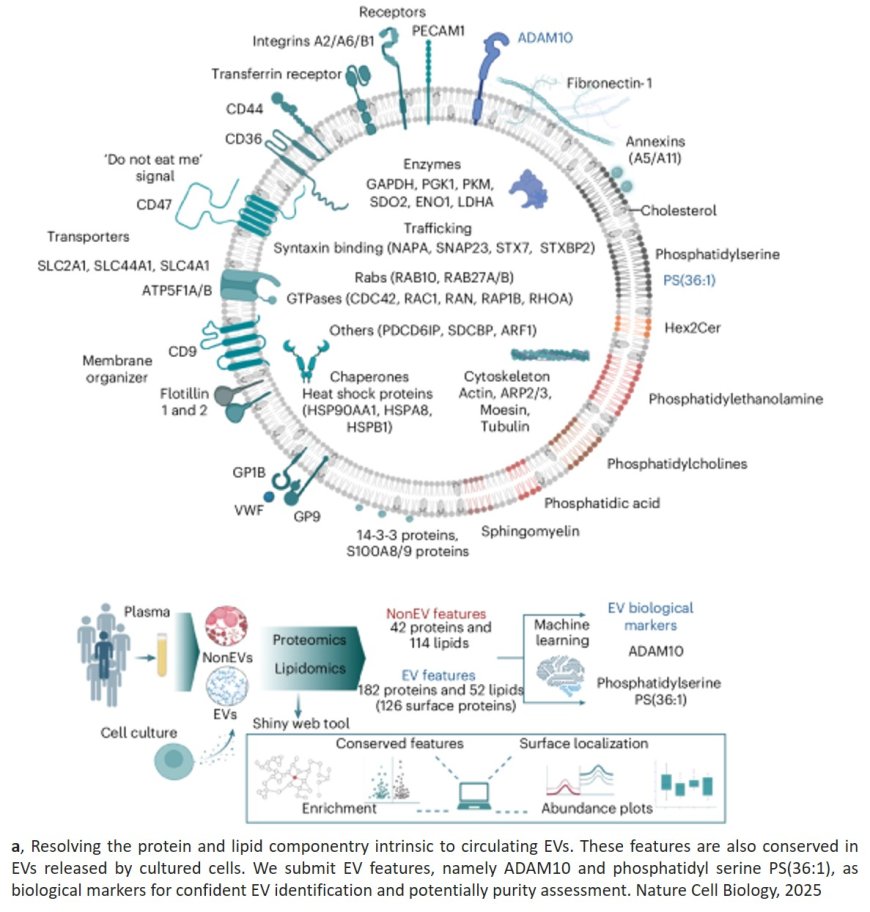
Using high-resolution density-gradient purification combined with integrated multi-omics profiling, the team achieved unprecedented molecular resolution, identifying 182 proteins (including ADAM10, STEAP23 and STX7) and 52 lipids (including PS, PIPs, Hex2Cer and PAs) that form the conserved “hallmark” features of plasma EVs.
They also mapped the surfaceome diversity, identifying 151 proteins on the EV surface. They also defined 29 proteins and 114 lipids characteristic of non-EV particles, creating a molecular benchmark to distinguish the two.
The authors identify ADAM10 and PS(36:1) as conserved EV biological markers that precisely differentiate between EV and non-EV particles.
This dataset provides a definitive molecular reference map for circulating EVs - a resource that will guide future studies of intercellular communication and disease biomarkers directly in humans. To ensure open access, the team developed EVMap.
The author said EVs were like biological emails, constantly sending molecular updates from one cell to another
“Until now, we couldn’t clearly read what those messages said. Using this platform, what we’ve uncovered is that their molecular ‘language’ can potentially identify patients with coronary artery calcification, an early sign of heart disease.” The author said.
“These signatures open the door to developing a simple blood test that could predict who is at highest risk of a heart attack years in advance.”
The team is now expanding its work through national collaborations to integrate EV proteome and lipidome data from large Australian population studies.
“This research represents years of persistence in refining EV isolation, mass-spectrometry workflows, and data analytics,” the author said. “It demonstrates how fundamental science can evolve into tools with real clinical impact.”