Liver identified as driver of body wasting in cancer
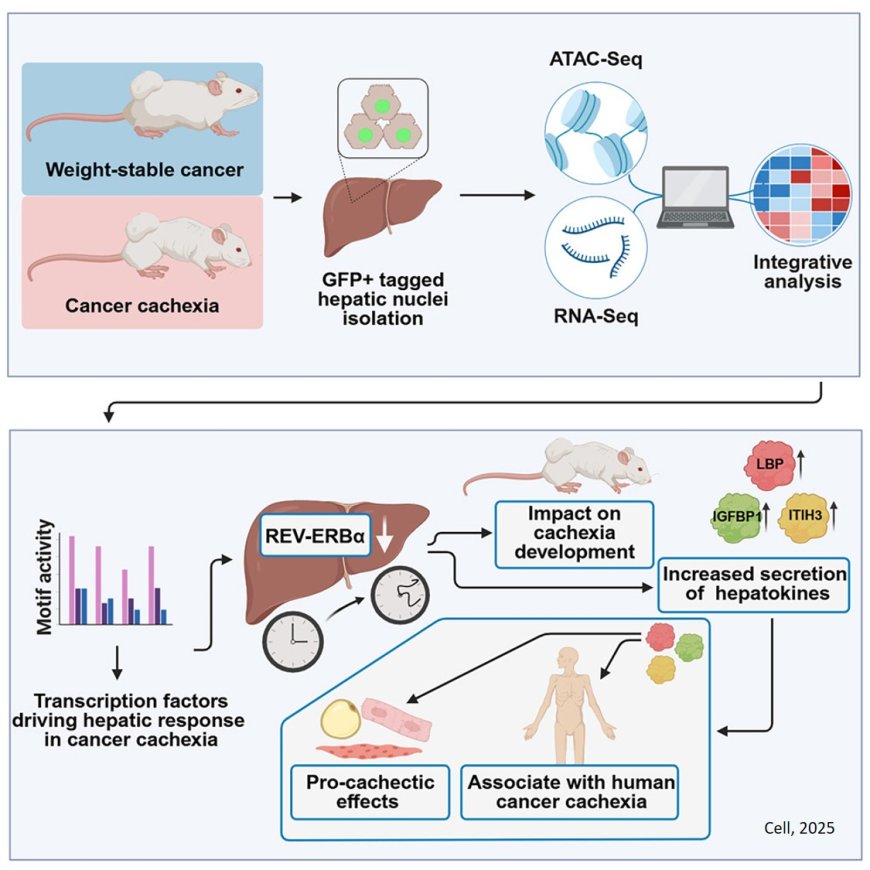
In cachexia, liver metabolism is fundamentally reprogrammed. One gene, which normally regulates the liver’s activity throughout the day, ceases to function properly. Using a mouse model, the researchers discovered that this "internal clock" was almost completely shut down.
“After reactivating the gene, known as REV-ERBα, specifically in the liver of affected mice, the loss of body mass was significantly reduced,” says a co-first author of the study.
The team showed that REV-ERBα regulates several genes involved in the production of liver-derived signaling molecules. When this clock gene is inactive, the liver releases increased levels of factors that promote disease progression. Three of these so-called hepatokines (LBP, ITIH3, and IGFBP1) are central to the newly discovered mechanism.
In cell culture experiments, they triggered catabolic, that is tissue-degrading, processes in muscle and fat cells, which are precisely the processes responsible for the physical wasting seen in cachexia. In addition, the levels of these proteins were markedly elevated in the blood of cachectic patients with various types of cancer. In preclinical models, targeted inhibition of these factors successfully mitigated their harmful effects.
“For the first time, we were able to show that the liver is not merely a passive responder to cachexia, but actively contributes to the progression of the disease,” says the study lead. “Our findings open up new possibilities to better diagnose the syndrome and explore therapeutic interventions.”
The study also provides a comprehensive data resource on the role of the liver in cachexia – ranging from molecular networks to cell type-specific changes and functional outcomes in preclinical models. This dataset is now available to the scientific community and can be used well beyond the model system studied.
In the long term, the identified factors could serve as biomarkers for cachexia risk or as targets for new therapies.