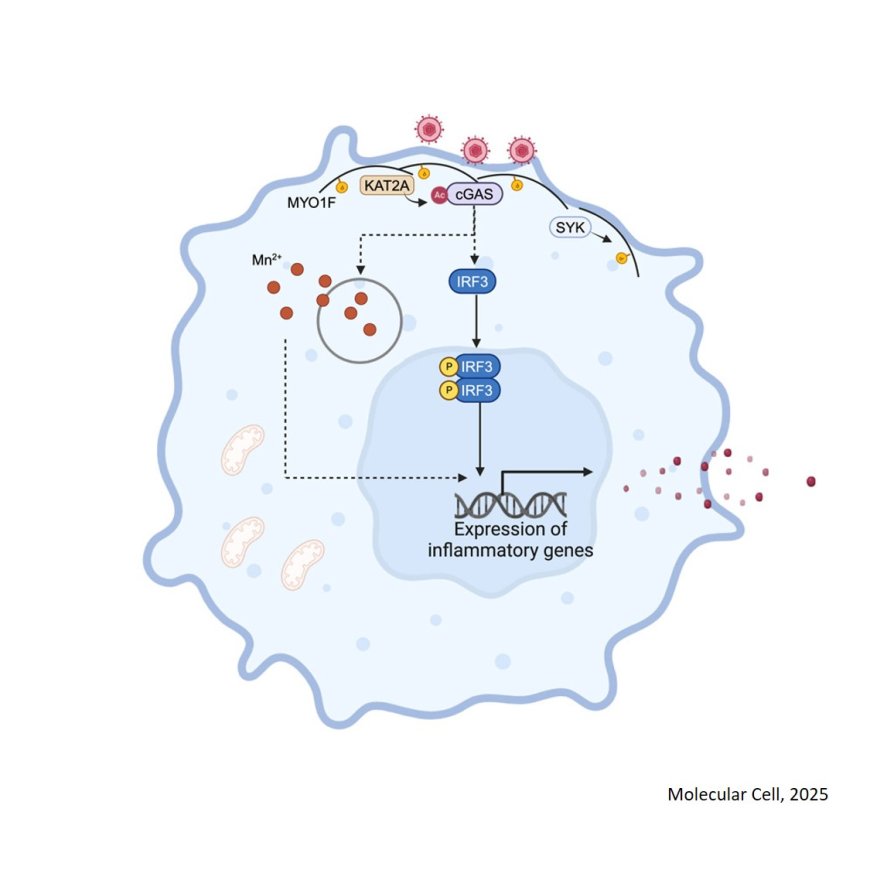
MYO1F positions cGAS on the plasma membrane to ensure full and functional signaling
Viral or endogenous DNA is detected by cyclic guanosine monophosphate (GMP)-adenosine monophosphate (AMP) synthase (cGAS), activating the innate immune response to infections and autoimmune diseases.cGAS binds to DNA and synthesizes 2′3′ cGMP-AMP, which triggers type I interferon production.
The appropriate subcellular localization of cGAS is crucial for its function and is found both in the cytosol and at the plasma membrane; however, the regulatory mechanisms and roles of membrane-localized cGAS remain largely unclear.
The researchers demonstrated that MYO1F facilitates the localization of cGAS to the plasma membrane, thereby influencing the execution of its function.
Mechanistically, phosphorylation of MYO1F by spleen-associated tyrosine kinase (SYK) during viral infection, facilitates the recruitment of lysine acetyltransferase 2A (KAT2A), which acetylates cGAS at lysine residues 421, 292, and 131, essential for its activation. Moreover, membrane-localized cGAS is crucial for signaling activation and type I interferon production triggered by virus-cell fusion due to Mn2+ release from organelles.
https://www.cell.com/molecular-cell/fulltext/S1097-2765(24)00951-1