Stem cell models show epilepsy genes disrupt different brain regions
For families of children with severe epilepsy, controlling seizures is often just the beginning of their challenges. Even in cases where powerful medications can reduce seizures, many children continue to face difficulties with learning, behavior and sleep that can be just as disruptive to daily life.
New stem cell-based research published in Cell Reports, provides an early step toward understanding why current treatments often fall short, pointing to the distinct effects that single disease-causing gene variants can have across different regions of the brain.
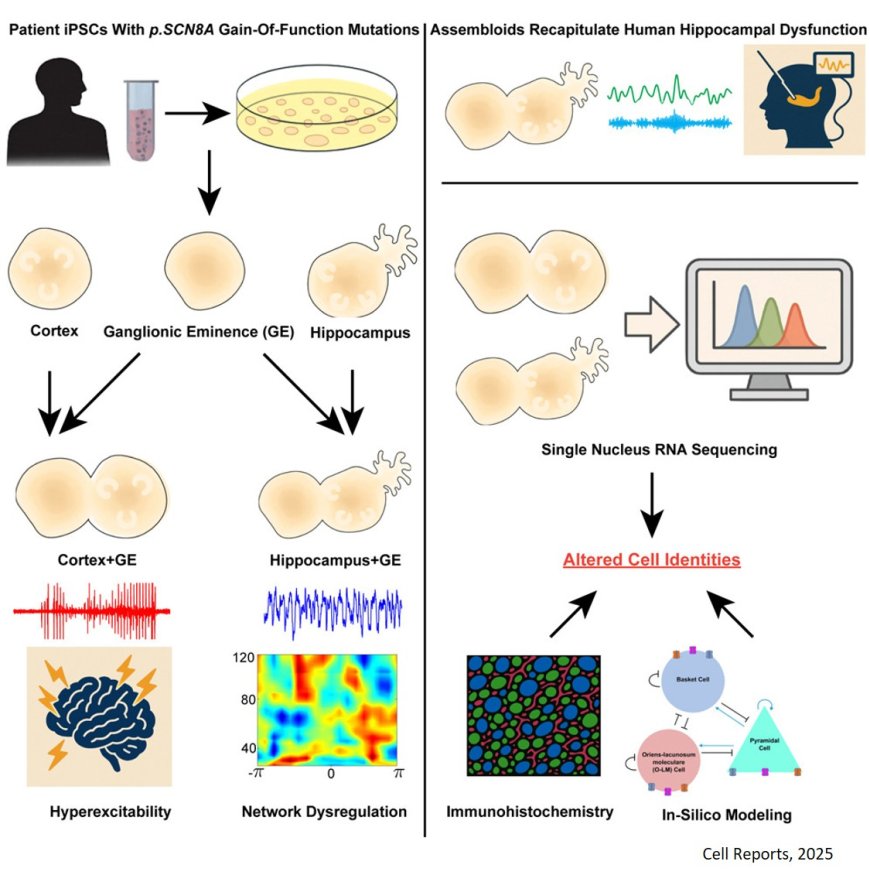
The study focuses on developmental and epileptic encephalopathy type 13, or DEE-13, a rare childhood condition caused by certain variants in the SCN8A gene. SCN8A encodes Nav1.6, a sodium channel critical for generating and transmitting electrical signals in neurons. Children with DEE-13 experience frequent seizures as well as developmental delays, intellectual disability, and autism spectrum disorder.
Using patient-derived induced pluripotent stem cells, the researchers generated advanced models known as 3D assembloids of two key brain areas: the cortex, which is essential for movement and higher-order thinking, and the hippocampus, which supports learning and memory. The results revealed strikingly different effects depending on the brain region.
In cortical models, the SCN8A variants made neurons hyperactive, mimicking seizure activity. In hippocampal models, however, the variants disrupted the brain rhythms associated with learning and memory. This disruption stemmed from a selective loss of specific hippocampal inhibitory neurons — the brain’s traffic cops that regulate neural activity.
These findings may help explain why patients with epilepsy often struggle with symptoms beyond seizures.
“Seizures are what bring families to the clinic, but for many parents, the bigger daily struggles are the other symptoms — problems with learning, behavior and sleep,” said a co-senior author. “What we found is that these cognitive problems aren’t just side effects of seizures. They likely arise from distinct disruptions in the hippocampus itself.”
Understanding these hippocampal disruptions is the first step toward identifying treatments that can help with the full range of symptoms, the author added.
To confirm their findings, the researchers compared brain recordings from people with epilepsy to stem cell-derived hippocampal assembloids. They looked at seizure-prone regions of the patients’ hippocampi as well as regions unaffected by seizures. Abnormal brain rhythms appeared in both the patients’ seizure “hot spots” and in assembloids carrying SCN8A variants. In contrast, seizure-free brain regions and assembloids without the variants showed normal activity.
“That was an important moment,” said the author. “It showed us that the disease processes we see in stem cell models mirror what happens in patients.”
Beyond its implications for epilepsy treatment, the study breaks new ground as the first to successfully create and characterize neural activity patterns in human hippocampal assembloids.
By demonstrating that stem cell-derived hippocampal tissue can generate authentic brain rhythms, the research provides a powerful new platform for investigating other conditions that affect learning and memory.
The technique could prove valuable for studying autism, schizophrenia and Alzheimer’s disease — all conditions where hippocampal function plays a crucial role.
“This is a foothold into a whole new area of research,” said a co-senior author. “We now have a system to ask how different diseases affect learning and memory circuits, and in the future to explore whether experimental therapies might improve brain activity in these models.”
https://www.cell.com/cell-reports/fulltext/S2211-1247(25)00988-X
https://sciencemission.com/Cortical-versus-hippocampal-network