Neuronal glycogen metabolism and tauopathy
A new study from scientists has revealed a surprising player in the battle against Alzheimer’s disease and other forms of dementia: brain sugar metabolism. Published in Nature Metabolism, the research uncovers how breaking down glycogen—a stored form of glucose—in neurons may protect the brain from toxic protein buildup and degeneration.
Glycogen is typically thought of as a reserve energy source stored in the liver and muscles. While small amounts also exist in the brain, particularly in support cells called astrocytes, its role in neurons has long been dismissed as negligible. “This new study challenges that view, and it does so with striking implications,” says the senior author of the study. “Stored glycogen doesn’t just sit there in the brain; it is involved in pathology.”
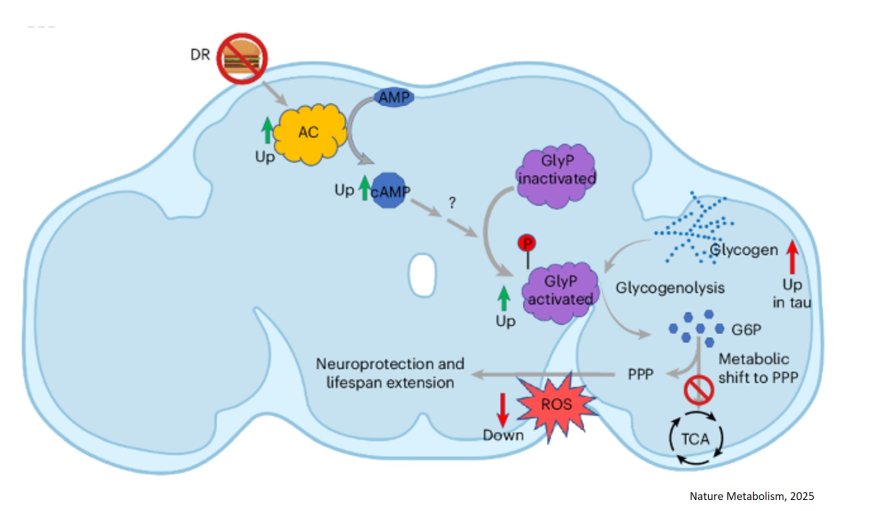
The research team discovered that in both fly and human models of tauopathy (a group of neurodegenerative diseases including Alzheimer’s), neurons accumulate excessive glycogen. More importantly, this buildup appears to contribute to disease progression. The author says tau, the infamous protein that clumps into tangles in Alzheimer's patients, appears to physically bind to glycogen, trapping it and preventing its breakdown.
When glycogen can’t be broken down, the neurons lose an essential mechanism for managing oxidative stress, a key feature in aging and neurodegeneration. By restoring the activity of an enzyme called glycogen phosphorylase (GlyP)—which kicks off the process of glycogen breakdown—the researchers found they could reduce tau-related damage in fruit flies and human stem cell-derived neurons.
Rather than using glycogen as a fuel for energy production, these enzyme-supported neurons rerouted the sugar molecules into the pentose phosphate pathway (PPP)—a critical route for generating NADPH (nicotinamide adenine dinucleotide phosphate) and Glutathione, molecules that protect against oxidative stress. “By increasing GlyP activity, the brain cells could better detoxify harmful reactive oxygen species, thereby reducing damage and even extending the lifespan of tauopathy model flies,” said the author.
Even more promising, the team demonstrated that dietary restriction (DR)—a well-known intervention to extend lifespan—naturally enhanced GlyP activity and improved tau-related outcomes in flies. They further mimicked these effects pharmacologically using a molecule called 8-Br-cAMP, showing that the benefits of DR might be reproduced through drug-based activation of this sugar-clearing system. “This work could explain why GLP-1 drugs, now widely used for weight loss, show promise against dementia, potentially by mimicking dietary restriction,” said the author.
Researchers also confirmed similar glycogen accumulation and protective effects of GlyP in human neurons derived from patients with frontotemporal dementia (FTD), strengthening the potential for translational therapies. The author says the study emphasizes the power of the fly as a model system in uncovering how metabolic dysregulation impacts neurodegeneration. “Work in this simple animal allowed us to move into human neurons in a much more targeted way,” the author said.
The author says this study not only highlights glycogen metabolism as an unexpected hero in the brain but also opens up a new direction in the search for treatments against Alzheimer’s and related diseases. “By discovering how neurons manage sugar, we may have unearthed a novel therapeutic strategy: one that targets the cell’s inner chemistry to fight age-related decline,” the author says. “As we continue to age as a society, findings like these offer hope that better understanding—and perhaps rebalancing—our brain’s hidden sugar code could unlock powerful tools for combating dementia”.