The gut immune system is altered in mouse model of Alzheimer’s
The gut contains the largest collection of immune cells in the body. New research shows that some of those immune cells travel along the brain/gut axis in a mouse model of Alzheimer’s disease (AD) providing a potential new therapeutic pathway for the memory-robbing malady. The research, published in Cell Reports, also shows that feeding the mice a high fiber diet reduces AD-related frailty, including tremor.
“This paper brings the gut immune system to the forefront of neurodegenerative disease pathology,” says co-senior author of the work. “Given its size and the cells’ ability to travel, it makes sense that those immune cells would have the ability to influence larger physiology.”
Another co-senior author adds, “As far as we know, this is the deepest investigation of the gut immune system in a model of neurodegenerative disease. We look forward to studying its impact in other diseases including Parkinson’s and multiple sclerosis.”
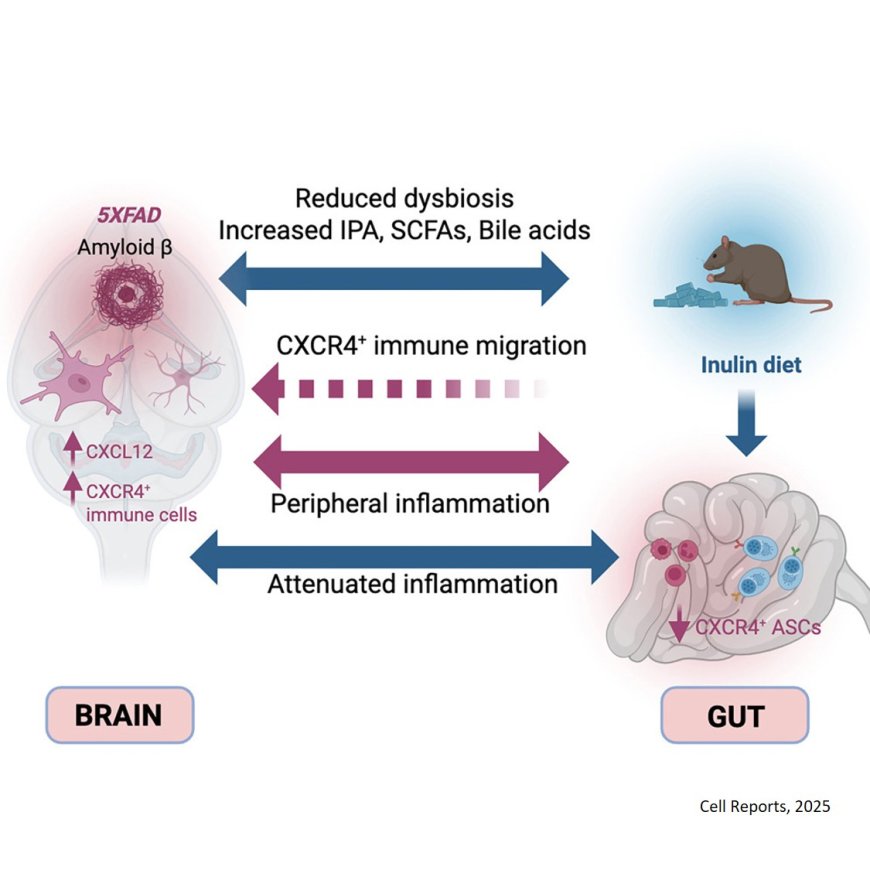
The authors found that specific antibody-producing B cells, normally responsible for keeping the microbiome and the gut immune system in harmony, were reduced in the mice bred to develop AD. They also discovered that this cell type has a migratory signature; researchers found the gut-specific B cells and their migratory receptors in the brain and in its border region, the meningeal dura mater. “Remarkably, we found that these immune cells in the brain border which recognize bacteria living in the intestines were accumulating in the AD brain,” the lead author says.
Wanting to understand what was driving the loss of immune cells in the gut, the team found that this gut immune cell receptor’s binding partner, a well-studied chemokine known for migration, was produced at higher levels in the glia, the inflammatory cells in the AD brain. The migratory signature was also identified in human AD brains via data mining of previously conducted studies.
Working with collaborators the team conducted blocking experiments in the axis using a small molecule drug, suggesting that a new long-range mechanism might be acting along the gut-brain axis.
The team found that feeding the animals the anti-inflammatory pre-biotic fiber inulin restored balance in the gut of the AD mice. “We found these migrating cells were replenished in the gut and that AD-related frailty, including the tremor trait, was reduced in the animals.” Noting that inulin makes short chain fatty acids and other metabolites that concentrate in the gut and can also circulate systemically, the author says the diet improved gut health and reduced chemokine signaling in the brain. “Again, this involved a bi-directional axis,” the author says.
The authors note that while the high fiber diet did not consistently reduce the levels of plaques in the mice’s brain, it did impact overall well-being. “We did an assay involving 31 metrics of aging in these mice. The diet definitely extended their healthspan, giving the animals a better quality of life,” another author says, adding, “This project supports the ‘eat your fruits and vegetables’ advice that is featured in nearly every dietary recommendation.”
While the study provides a comprehensive characterization of gut immune system changes in a neurological disease, researchers say more work is needed to see if those changes are a response to brain alterations or whether they drive the disease itself. The auhtor says one possibility is that age-related insults might trigger AD-causing inflammation in the brain with chemokines signaling the gut immune system for help in dealing with the insult. “In the beginning the process is likely protective, but over time the gut becomes compromised setting the stage for more dangerous types of bacteria to flourish which fuels inflammation throughout the body.”
The authors are eager to explore the potential of understanding and/or altering the gut microbiome in the context of disease. “Maybe there is a microbiome that signals an increased risk of neurological disease. Perhaps we’ll be able to identify specific bacteria that set off immune system inflammation. What if we can inhibit the signaling chemokines early versus late in the disease process? Which would be protective for the whole system? This paper provides so many avenues for further exploration.”
https://www.cell.com/cell-reports/fulltext/S2211-1247(25)00880-0