Significant differences in trypanosomal and human nuclear cap-binding complex
Trypanosomes are parasites that cause deadly diseases in humans and animals, such as Human African Trypanosomiasis (sleeping sickness), Chagas disease, and Nagana in cattle. According to the World Health Organisation, sustained control efforts have reduced the number of sleeping sickness infections, but millions of people are still estimated to be infected with Chagas disease. Diagnosis and treatment remain complex, and no effective vaccine has been developed for these neglected tropical diseases.
A new study from the research group characterized the structure of an important trypanosomal protein complex, potentially paving the way for novel drug discovery in the future.
Currently, the insect species that transmit trypanosomes are mostly found in the Southern Hemisphere. However, as climate change may increase the spread of insect-borne diseases across the globe, understanding the biology of these parasites is becoming even more urgent.
Trypanosomes – like other living organisms – transcribe their DNA into messenger RNAs (mRNAs), which serve as a set of instructions for the cells to build proteins, which are essential for nearly all processes in living organisms. They perform specific biological tasks, such as the infection process in the host.
Both humans and trypanosomes are eukaryotic organisms – those that pack their DNA into a nucleus. In eukaryotes, mRNAs undergo extensive processing before the instructions they carry can be used by the cell. Since the processing of human and trypanosome mRNAs bears significant differences, a better understanding of these molecular processes is fundamental to developing drugs that specifically inhibit RNA processing in the parasite without affecting human cells.
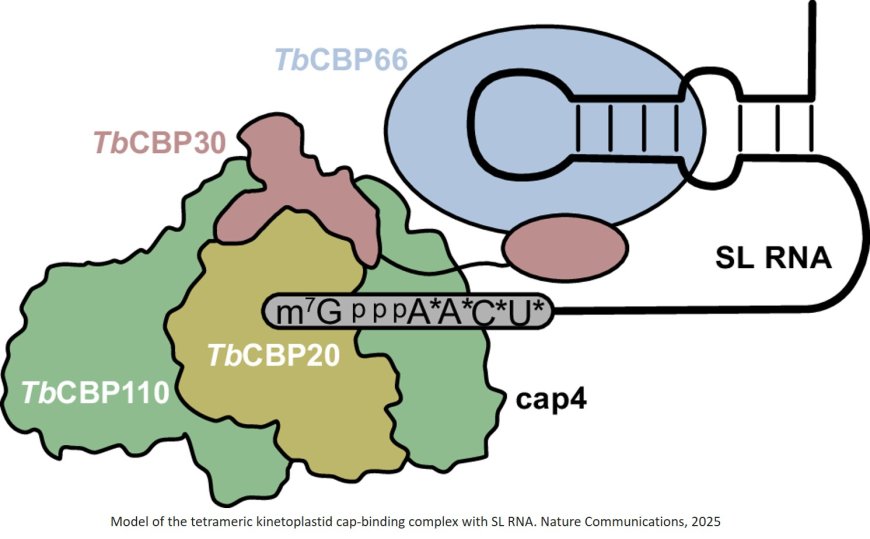
In their recent study, published in Nature Communications, the group characterized the nuclear cap-binding complex. This complex binds all mRNAs in trypanosomes and is vital for correct mRNA processing and thus for the survival of the parasite. The researchers reveal major differences between the trypanosomal and human nuclear cap-binding complex, suggesting it could serve as a potential drug target.
In all eukaryotes, the nuclear cap-binding complex is a key player in cellular RNA metabolism. It binds RNA at a very early stage during its production. Other components (called factors) of the cellular machinery then interact with the newly synthesized RNA molecule via the cap-binding complex. These factors use the cap-binding complex as a platform to lead the RNA to its next processing step or to a new location in the cell.
Unlike its human counterpart, which has two sub-units, the trypanosome nuclear cap-binding complex consists of four subunits. The function of three of them had not been understood so far – which triggered the interest of the group.
Their research revealed that the complex consists of two lobes – one that resembles the human complex and a second part that is connected to the first through a very flexible protein.
“A major challenge of studying this complex was its flexibility,” said the first author of the study. “I was using cryo-electron microscopy (cryo-EM) to generate a structure of the complex, but the flexible parts were never resolved in our data. We thus turned to small-angle X-ray scattering.” The researchers used the X-ray beams available at the European Synchrotron Radiation Facility on Grenoble’s European Photon and Neutron science campus to assess the flexible parts of the complex.
The end of mRNA molecules usually carries a chemical modification, but in trypanosomes, this so-called ‘cap’ is hypermodified, with many more modifications than in other organisms. “Since trypanosomes carry a special RNA cap structure, we were also interested in how exactly the nuclear cap-binding complex interacts with this cap and the RNA molecule in general,” added the principal investigator on this project.
The researchers used their cryo-EM structures to reveal the interaction between the nuclear cap-binding complex and the RNA cap structure. Binding assays also suggested a second, novel, binding site for RNA in the complex. “We followed up on this observation and determined that one of the flexibly linked subunits, whose function was previously unknown, specifically binds double-stranded RNAs,” explained an author.
Because it is essential for the parasite, but very different from the human complex, the cap-binding complex could be a future drug target.
Additionally, the data on the nuclear cap-binding complex will serve as the foundation to fully understand the details of RNA biogenesis and processing in Trypanosoma brucei (T.brucei). In addition to its impact on human and animal health, T. brucei is also used as a model organism in research to explore the origins of eukaryotic life and evolution.
https://www.nature.com/articles/s41467-024-55373-w
https://sciencemission.com/Trypanosoma-brucei-cap-binding-complex