Key lung immune cells can intensify allergic reactions
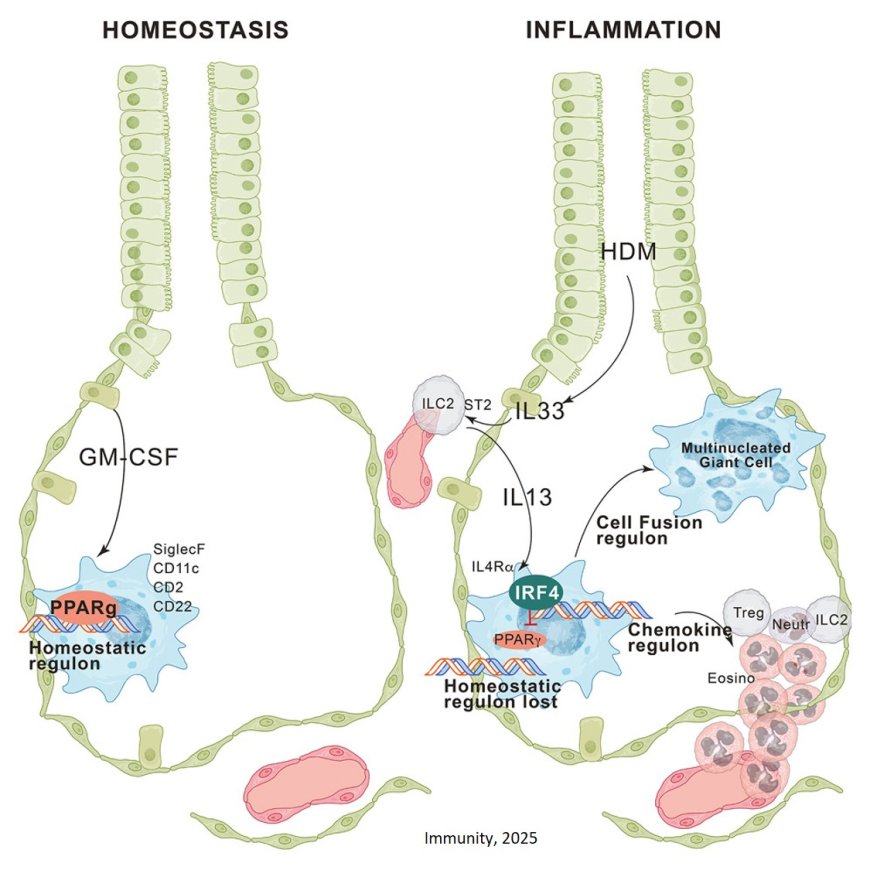
Alveolar tissue resident macrophages (trAMs) are immune cells that live in the tiny air sacs of the lungs. Under normal conditions, these cells act as guardians, keeping the lungs healthy, supporting breathing, and preventing unnecessary immune responses.
However, new work shows that during allergic reactions, these macrophages can undergo a dramatic change. Instead of calming the immune system, they switch into an inflammatory mode that actively fuels allergy-driven lung inflammation.
“Alveolar macrophages have long been seen as peacekeepers in the lung,” said the first author of the study. “Our results show that during allergic responses, they can do the opposite and actually help drive inflammation.”
Using an advanced mouse model that allowed the researchers to precisely track and manipulate these lung cells, the team discovered that allergen exposure causes alveolar macrophages to send out signals that attract other immune cells into the lung. This influx amplifies inflammation and worsens allergic reactions. Remarkably, the macrophages were also found to fuse together into large ‘giant cells’ that change the structure of the lung tissue during allergy.
Mechanistically, upon allergen exposure, interleukin-33-activated innate type 2 lymphoid cells (ILC2s) produced interleukin-13, which reprogrammed trAMs through induction of the transcription factor interferon regulatory factor 4 (IRF4).
IRF4 suppressed the expression of the transcription factor peroxisome proliferator-activated receptor gamma (PPARγ) and dismantled the PPARγ-dependent homeostatic regulon that defines trAM identity, while initiating a transcriptional program driving chemokine production and cell fusion. This resulted in the recruitment of granulocytes, ILC2s, and regulatory T cells, as well as the formation of multinucleated giant cells in the alveolar niche.
These findings challenge the long-standing view that alveolar macrophages are stable cells that resist change. Instead, the study reveals that they are surprisingly flexible and can be reprogrammed by their environment, sometimes with harmful consequences.
The discovery has important implications for understanding allergic lung diseases such as asthma. Current treatments mainly target other immune cells or inflammatory molecules. By identifying alveolar macrophages as active drivers of allergic inflammation, the study opens new avenues for research into therapies that could prevent or reverse their harmful switch, potentially reducing inflammation while preserving essential lung function.
Together, the results offer a new perspective on how allergic reactions escalate in the lung and highlight the complex, double-edged role of immune cells that are essential for health—but, under the wrong conditions, can contribute to disease.
https://www.cell.com/immunity/abstract/S1074-7613(25)00517-5