Dysregulation of mTOR signalling is a converging mechanism in lissencephaly
Lissencephaly is a spectrum of rare, genetic disorders in which the brain fails to develop its hallmark folds. The disorders are often associated with seizures and intellectual disability and currently there are no available treatments.
A new study, however, has identified a molecular mechanism that underlies some lissencephaly disorders — and a drug that prevents and reverses lissencephaly malformations in organoids (small, three-dimensional replicas of developing brains that allow scientists to study early brain development).
The findings, published in the journal Nature, may point to a target for treatment, researchers say.
“Lissencephaly belongs to a group of disorders we call malformations of cortical development, meaning the normal development and structure of the brain is disrupted,” said a co-senior author of the study. “They come about because certain genes that are very important for brain development are affected by rare mutations.”
Past research has connected a number of genes to lissencephaly, but there are some patient cases where the genetic causes remain unknown. Further, how these genetic mutations actually lead to lissencephaly at the molecular level has not been well understood.
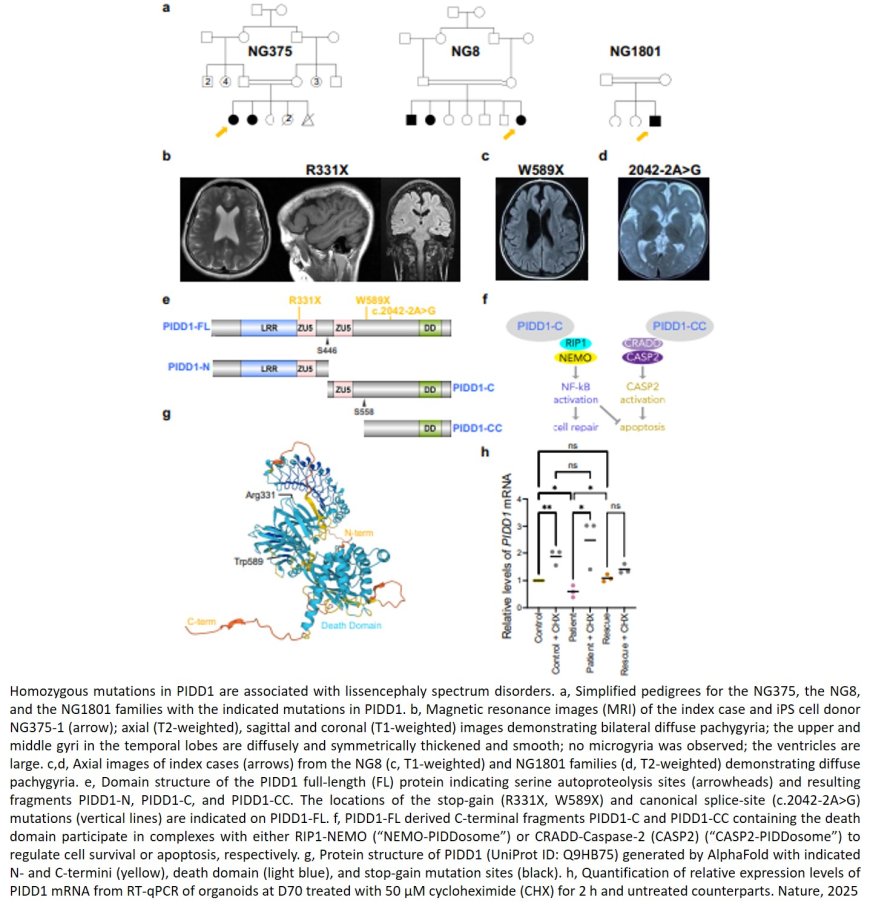
For the new study, researchers found a new gene associated with lissencephaly and then developed brain organoids from the cells of patients with two different types of lissencephaly. Specifically, they took cells from strands of the patients’ hair and, through a chemical method, reversed the cells’ development, pushing them into an earlier, unspecialized cell stage. The researchers then reprogrammed the cells to become neurons, which grew together to form three-dimensional organoids.
Along with having little to no folding in their brains, individuals with lissencephaly also have a thicker than usual cerebral cortex. The organoids grown from the patients’ cells for the new study also developed thicker cortex-like structures than healthy organoids, much like what’s observed in lissencephaly, researchers found.
The research team also performed several analyses to evaluate the gene and protein expression levels in the organoids. Their findings pointed to dysregulation in the mTOR (mammalian target of rapamycin) pathway in both types of lissencephaly organoids they were studying.
“This is a fundamental pathway that governs many different aspects of cellular metabolism to maintain cellular homeostasis,” said the author. “And we know of many disorders in which the mTOR pathway is overactive, but here we found that in lissencephaly it’s actually underperforming.”
Ultimately, the researchers exposed the organoids to a drug that boosts mTOR pathway activity and found that it could prevent and reverse thickening of the organoid cortical plate-like area depending on when it was introduced.
“Right now, in medicine we have no way to slow or reverse these structural brain malformations in lissencephaly either during pregnancy or after,” said the lead author. “That limits us to treating the symptoms, but even that can be difficult, as lissencephaly seizures may not be well-controlled using typical anti-epileptic drugs.”
But since the study showed that the mTOR pathway is implicated in two different types of lissencephaly, it suggests this might be the case for additional types of lissencephaly — or maybe even the entire spectrum of lissencephaly disorders.
“If there’s a converging pathway shared between these disorders, regardless of the genetic cause, it could mean one treatment, such as a mTOR activator like the one we tested in the study, might be beneficial to patients across the lissencephaly spectrum,” said the author.
Going forward, the researchers aim to determine whether the mTOR pathway is implicated in other genetic types of lissencephaly and dig deeper into how an underactive mTOR pathway leads to lissencephaly.
“These findings extend our knowledge of this pathway, highlighting the fine balance that has to be met for healthy brain development,” said the author. “Now we want to understand what exactly happens molecularly when mTOR is underactivated.”
It will be important to explore potential clinical applications of mTOR activators in this spectrum of disorders as well, the author added, as benefiting patients though basic discoveries is the program’s ongoing motivation.