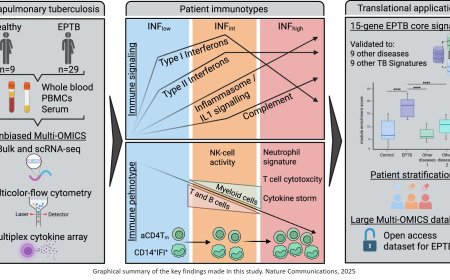
How microbiota protects against influenza in newborns

In 2017, scientists revealed that using antibiotics to protect newborns from dangerous infections often comes with a long-term consequence—a permanently underdeveloped immune system that can make children prone to poor outcomes from future lung infections.
Now a study published in Cell, details the mechanisms behind antibiotic-related immune disruptions, which in turn suggests a way to reverse or minimize the risk.
"These remarkable findings indicate that we might be able to protect at-risk infants through targeted supplementation," says senior author. “Our team tested a supplement that achieved positive results in mice, but this would require more testing and confirmation through human clinical trials before any clinical recommendations could be made.”
The authors found that antibiotic-exposed infants develop fewer specialized "memory" T cells in their lungs—the immune system's frontline defenders against respiratory infections.
"We've discovered that the gut microbiome acts as a teacher for the developing immune system," the author says.
"When antibiotics disrupt this natural education process, it's like removing key chapters from a textbook. The immune system never learns crucial lessons about fighting respiratory infections," the author adds.
The study compared data from mouse and human infants exposed to ampicillin, gentamicin, and vancomycin—all antibiotics frequently used in pregnant women and newborns—with those who maintained their natural gut bacteria. The differences were striking:
· Antibiotic-exposed mouse and human infants had significantly reduced populations of protective CD8+ T cells in their lungs
· These infants showed impaired ability to form "tissue-resident memory cells," specialized immune cells that live in the lungs and provide rapid protection against reinfection
· In a mouse model, the immune deficits persisted into adulthood, suggesting permanent changes to immune development
The research team identified a specific mechanism linking gut bacteria to lung immunity. They found that Bifidobacterium, a species of beneficial bacteria commonly found in healthy infant guts, produces a molecule called inosine. This metabolite acts as a critical signal for proper immune cell development.
"Think of inosine as a molecular messenger," the senior author says. "It travels from the gut to developing immune cells, telling them how to mature properly and prepare for future infections."
When antibiotics eliminate these beneficial bacteria, inosine levels plummet, and immune cells fail to receive proper developmental signals. The team discovered that this disruption affects a master regulator protein called NFIL3, which controls how T cells mature and function.
Importantly, the authors validated their findings in human infants. By analyzing lung tissue from infants who had died from various causes, they confirmed that antibiotic-exposed human babies showed the same immune deficits as those observed in mice.
The antibiotic-exposed infants had fewer influenza-specific memory T cells in their lungs and a reduced ability to mount effective immune responses when challenged with viral proteins. Their tissues also showed similar gene expression patterns to those seen in elderly individuals, who also are vulnerable to respiratory infections.
Perhaps most excitingly, when the team supplemented antibiotic-exposed infant mice with inosine, they observed significant restoration of immune function. The treatment:
· Restored normal T cell development patterns
· Improved the formation of protective memory cells
· Enhanced resistance to influenza infection
· Reduced illness severity when infections did occur
The researchers emphasize that antibiotics remain life-saving medications that should be used when medically necessary.
“However, these findings suggest that clinicians should be judicious about antibiotic use during pregnancy and early infancy and consider probiotic or prebiotic interventions to support healthy microbiome development,” the senior author says.
Meanwhile, more research is needed to explore the potential value of inosine supplementation for at-risk infants and to develop other strategies to protect antibiotic-exposed infants from future respiratory infections.