New mRNA cancer vaccine triggers fierce immune response to fight malignant brain tumor
In a first-ever human clinical trial of four adult patients, an mRNA cancer vaccine by reprogramming immune system to attack glioblastoma, the most aggressive and lethal brain tumor.
The results mirror those in 10 pet dog patients suffering from naturally occurring brain tumors whose owners approved of their participation, as they had no other treatment options, as well as results from preclinical mouse models. The breakthrough now will be tested in a Phase 1 pediatric clinical trial for brain cancer.
Reported in the journal Cell, the discovery represents a potential new way to recruit the immune system to fight notoriously treatment-resistant cancers using an iteration of mRNA technology and lipid nanoparticles, similar to COVID-19 vaccines, but with two key differences: use of a patient’s own tumor cells to create a personalized vaccine, and a newly engineered complex delivery mechanism within the vaccine.
“Instead of us injecting single particles, we’re injecting clusters of particles that are wrapping around each other like onions, like a bag full of onions,” said the senior author. “And the reason we’ve done that in the context of cancer is these clusters alert the immune system in a much more profound way than single particles would.”
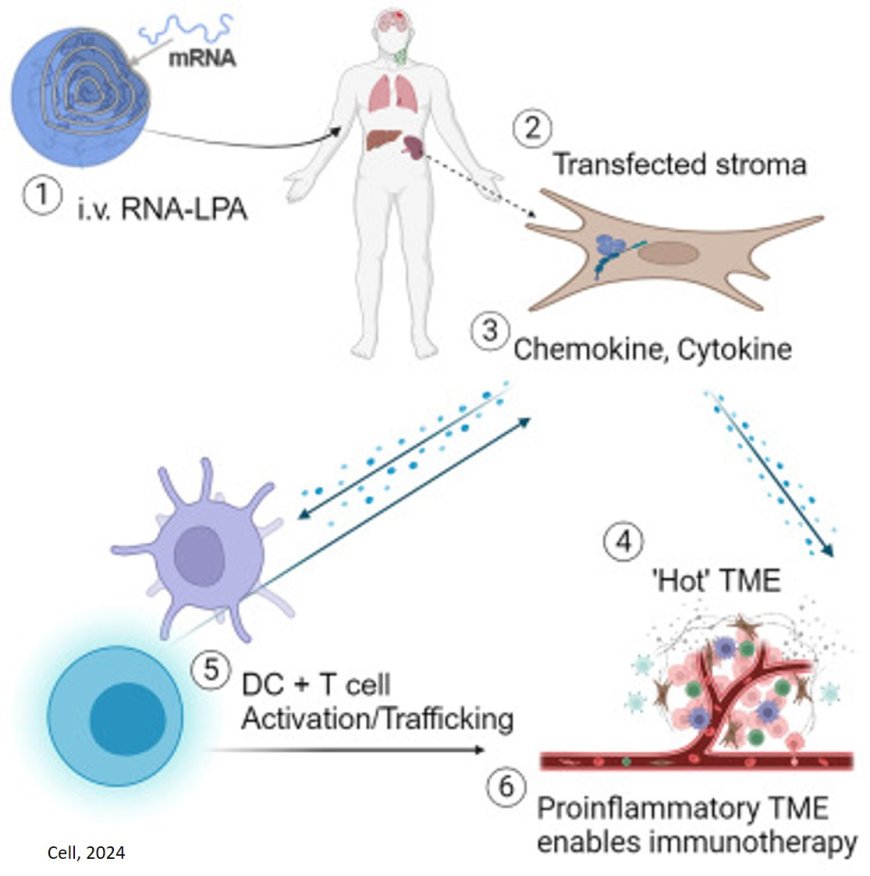
Among the most impressive findings was how quickly the new method, delivered intravenously, spurred a vigorous immune-system response to reject the tumor, said the principal investigator.
“In less than 48 hours, we could see these tumors shifting from what we refer to as ‘cold’ — immune cold, very few immune cells, very silenced immune response — to ‘hot,’ very active immune response,” the author said. “That was very surprising given how quick this happened, and what that told us is we were able to activate the early part of the immune system very rapidly against these cancers, and that’s critical to unlock the later effects of the immune response.”
Glioblastoma is among the most devastating diagnoses, with median survival around 15 months. Current standard of care involves surgery, radiation and some combination of chemotherapy.
The new publication is the culmination of promising translational results over seven years of studies, starting in preclinical mouse models and then in a clinical trial of 10 pet dogs that had spontaneously developed terminal brain cancer and had no other treatment options. That trial was conducted with owners’ consent.
Dogs offer a naturally occurring model for malignant glioma because they are the only other species that develops spontaneous brain tumors with some frequency, said a co-author. Gliomas in dogs are universally terminal, the co-author said.
After treating pet dogs that had spontaneously developed brain cancer with personalized mRNA vaccines, the team advanced the research to a small Food and Drug Administration-approved clinical trial designed to ensure safety and test feasibility before expanding to a larger trial.
In a cohort of four patients, genetic material called RNA was extracted from each patient’s own surgically removed tumor, and then messenger RNA, or mRNA — the blueprint of what is inside every cell, including tumor cells — was amplified and wrapped in the newly designed high-tech packaging of biocompatible lipid nanoparticles, to make tumor cells “look” like a dangerous virus when reinjected into the bloodstream and prompt an immune-system response. The vaccine was personalized to each patient with a goal of getting the most out of their unique immune system.
“The demonstration that making an mRNA cancer vaccine in this fashion generates similar and strong responses across mice, pet dogs that have developed cancer spontaneously and human patients with brain cancer is a really important finding, because oftentimes we don’t know how well the preclinical studies in animals are going to translate into similar responses in patients,” said a co-author of the paper. “And while mRNA vaccines and therapeutics are certainly a hot topic since the COVID pandemic, this is a novel and unique way of delivering the mRNA to generate these really significant and rapid immune responses that we’re seeing across animals and humans.”
While too early in the trial to assess the clinical effects of the vaccine, the patients either lived disease-free longer than expected or survived longer than expected.
The 10 pet dogs lived a median of 139 days, compared with a median survival of 30 to 60 days typical for dogs with the condition.
The next step, through support from the Food and Drug Administration and the CureSearch for Children’s Cancer foundation, will be an expanded Phase I clinical trial to include up to 24 adult and pediatric patients to validate the findings. Once an optimal and safe dose is confirmed, an estimated 25 children would participate in Phase 2, said a co-author.
Despite the promising results, the authors said one limitation is continued uncertainty about how best to harness the












