The first image of an open NMDA receptor
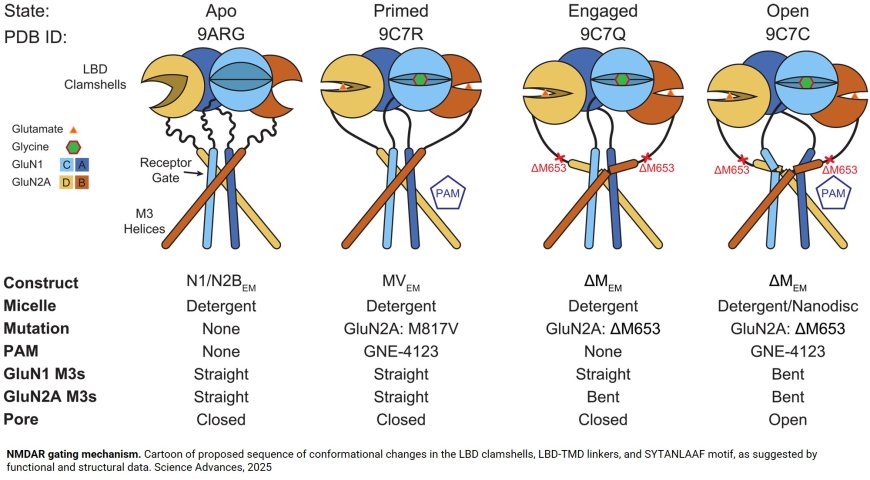
When it comes to brain proteins, small changes can make a dramatic difference. Researchers studying NMDA (N-methyl-D-aspartate) receptors, which are essential for learning, memory and moment-by-moment consciousness, know that even slight changes in their activity level can mean the difference between normal function and serious neurological disorders.
Now, researchers have captured for the first time and in exquisite detail pictures of receptors in a fully open conformation. The paper also describes for the first time the sequence of structural changes that transform receptors from being silent to being fully active.
Published today in Science Advances, the team’s work expands the understanding of how NMDA receptors function and how their malfunction contributes to disease.
“NMDA receptors are key to ongoing brain activity,” the corresponding author says, “but when they are inappropriately active, they kill neurons, such as, for example, after a traumatic brain injury, after a stroke, or during progressive neurodegenerative diseases like Parkinson’s disease and Alzheimer’s disease.”
For that reason, the authors have been focused on trying to understand and track the sequence of events that transform resting, inactive receptors into active, electricity-generating receptors. A key part of assembling this sequence requires obtaining pictures of receptors in different functional states to identify which parts change position during activation, enabling the transition. While previous research has reported atomic-resolution structures for receptors in the resting state, the author says that structures for active, pore-open receptors have remained elusive.
The researchers used cryogenic electron microscopy (cryo-EM), which involves flash-freezing samples of the receptors, then probing them with an electron beam to get a 3D view. The result is the first-ever, high-resolution image of a stable, open NMDA receptor. Unlike closed-pore receptors, in which the pore’s four spiral structures, or helices, are straight, the researchers found that when the pore opens, the helices are bent or kinked.
It wasn’t what they expected. “We thought that the channel would open simply by prying apart the four helical rods that prevent ion flow to widen the mouth of the pore,” says the author. “Instead, we saw that all four rods are kinked outward and stabilized in this splayed conformation by new contacts with distant parts of the molecule.”
Each kink, the author explains, represents a region of interest in the receptor. “The identity of the amino acid at that position is essential as it must allow the kinking,” the author says. “Without the image, without knowing that the helix must bend, we would not understand why this particular residue is important.”
The new observations will help researchers figure out the step-by-step process by which the receptor pore opens; they also provide insight into which other parts of the receptor may also play a role. There are many patients, the author notes, with single-point mutations in the DNA coding for this receptor. A point mutation is a change in a single amino acid in the receptor’s structure. “The new information helps us better understand what these amino acid residues do, why they are important and what goes wrong when they are altered,” the author adds.
The researchers also reveal the first mechanism of NMDA receptor activation integrating functional evidence obtained in the lab from single-molecule electrophysiology recordings with structural evidence from single-particle cryo-EM.
“Before this study, we knew that the receptor must change its structure several times to transition from resting to primed to open,” says the author. “Separately, structural studies produced pictures of receptors that looked a little different, but because we didn’t know how the final open state looked, we couldn’t arrange the pictures in the correct sequence.”
https://www.science.org/doi/10.1126/sciadv.adx4647
https://sciencemission.com/Cryo-EM-snapshots-of-NMDA-receptor