Dual nature of polyamines in longevity to cancer
Polyamines, a group of naturally occurring molecules found in all living organisms, are essential for fundamental cellular processes, such as growth and differentiation. In recent years, these compounds (particularly spermidine) have gained attention as promising ‘geroprotectors’ that promote healthy aging and extend lifespan.
Studies have shown that polyamines can activate beneficial cellular processes like autophagy, which helps clear damaged cell components, primarily through a protein called eukaryotic translation initiation factor 5A (eIF5A1). However, these positive effects are overshadowed by a troubling paradox, as elevated polyamine levels are also consistently observed in various cancers and are associated with rapid tumor growth.
Despite the known link between polyamines and cancer, the exact molecular mechanisms by which these compounds directly drive cancer progression remain unclear. Cancer cells are known for their unique metabolic adaptations, such as their higher reliance on aerobic glycolysis, but how polyamines specifically contribute to these changes remains largely unknown. On top of this, while eIF5A1 has well-studied roles in healthy cells, its closely related counterpart eIF5A2 appears to be involved in oncogenesis. How can these highly similar proteins, which share 84% of their amino acid sequence, have such different effects?
To address these questions, a research team conducted a comprehensive study using advanced proteomic and molecular techniques. Their findings were published in the Journal of Biological Chemistry, shed light on how polyamines promote cancer cell growth through molecular pathways distinct from those linked to their beneficial effects in healthy aging.
The researchers used human cancer cell lines to investigate how polyamines influence protein production and cellular metabolism. By depleting polyamines with a drug and then restoring them manually via spermidine supplementation, the researchers could observe the specific effects of these molecules on cancer cell behavior.
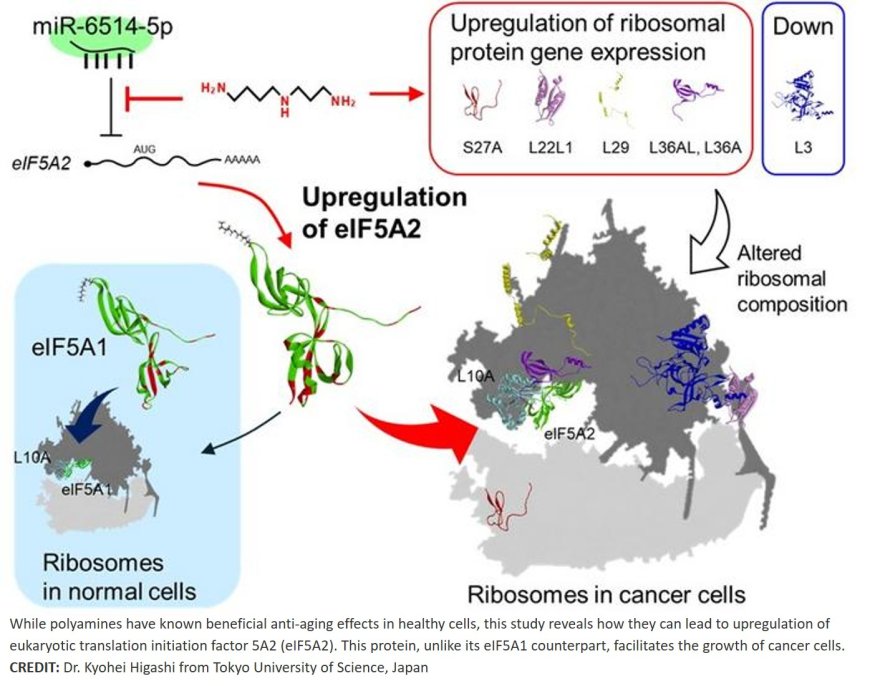
To this end, they employed cutting-edge proteomic analysis techniques to examine changes in over 6,700 proteins. They found that polyamines primarily activate glycolysis—a metabolic pathway that rapidly converts glucose to energy—rather than the mitochondrial respiration pathways associated with healthy aging. Furthermore, polyamines upregulate the expression of eIF5A2 and five ribosomal proteins, particularly RPS 27A, RPL36AL, and RPL22L1, which are associated with cancer malignancy.
A comparative analysis of the functions of eIF5A1 and eIF5A2 and their interactions with polyamines yielded key findings. “The biological activity of polyamines via eIF5A differs between normal and cancer tissues,” explains the author. “In normal tissues, eIF5A1, activated by polyamines, activates mitochondria via autophagy, whereas in cancer tissues, eIF5A2, whose synthesis is promoted by polyamines, controls gene expression at the translational level to facilitate the proliferation of cancer cells.”
The researchers conducted further experiments to pinpoint the precise mechanism by which polyamines stimulate eIF5A2 production. They discovered that the initiation of eIF5A2 protein synthesis is normally suppressed by a small regulatory RNA molecule called miR-6514-5p. Polyamines interfere with this suppression, allowing eIF5A2 levels to increase. Furthermore, their analysis revealed that eIF5A2 regulates a completely different set of proteins compared to eIF5A1.
These discoveries have important implications for both cancer treatment and the safe use of polyamine supplements. The results suggest that context matters enormously, and that while polyamines may offer anti-aging benefits in healthy tissues through eIF5A1, they can promote cancer growth through eIF5A2 in potentially malignant tissues. This dual nature explains why polyamines have been such a puzzle in medical research.
Notably, this study highlights a novel mechanism involved in cancer growth that could be exploited in oncology drug development. “Our findings reveal an important role for eIF5A2, regulated by polyamines and miR-6514-5p, in cancer cell proliferation, suggesting that the interaction between eIF5A2 and ribosomes, which regulates cancer progression, is a selective target for cancer treatment,” remarks the author. In principle, it may be possible to leverage this newfound knowledge to develop targeted cancer therapies that specifically inhibit eIF5A2 function without affecting beneficial eIF5A1 pathways.
Overall, this work represents a significant step forward in understanding the seemingly paradoxical properties of polyamines. In the future, these findings could lead to novel strategies that harness the positive effects of these molecules while minimizing their cancer-related risks.
https://www.jbc.org/article/S0021-9258(25)02303-8/fulltext
https://sciencemission.com/Polyamines-stimulate-the-protein-synthesis