Small molecule shows early-stage promise for repairing myelin sheath damage
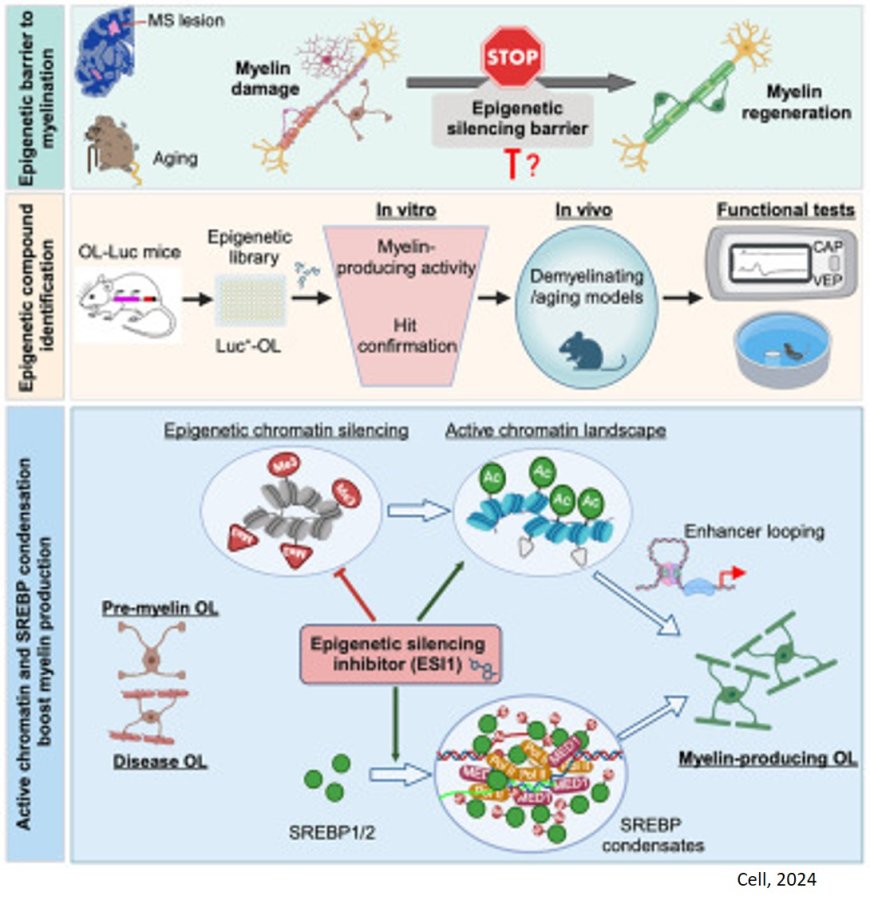
When treated with a novel protein function inhibitor called ESI1, mice that mimic the symptoms of multiple sclerosis (MS) and lab-prepared human brain cells both demonstrated the ability to regenerate vital myelin coatings that protect healthy axon function.
This breakthrough, published in Cell, appears to overcome difficulties that have long frustrated previous attempts to reverse a form of nerve damage that robs people with MS of motor control and gradually blunts cognitive functions for many people as they age.
“Currently, there are no effective therapies to reverse myelin damage in devastating demyelinating diseases such as MS,” says the corresponding author. “These findings are significant as they offer new pathways for treatment that potentially shift the therapeutic focus from just managing symptoms to actively promoting repair and regeneration of myelin.”
A critical insight driving the new findings was observing that brain regions damaged by MS still possessed a type of cell needed to repair myelin damage, but the disease activates other cell types and signals that combine forces to silence the repair function.
These useful cells in the brain, called oligodendrocytes, are responsible for producing myelin sheaths that wrap around cable-like parts of nerve cells called axons, much like the plastic insulation around a wire. When the protective myelin gets damaged, be it by disease or the wear and tear of age, nerve signaling gets disrupted. Depending on where the damaged nerves lead, the disruptions can affect movement, vision, thinking and so on.
Essentially, the research team found a way to unsilence the silenced repair process, setting the oligodendrocytes (OLs) free to do their jobs.
Pinning down the genetic changes and signals involved in the repair silencing process and finding a small molecule compound that can reverse the silencing was a complex undertaking.
Analysis of stored autopsy tissues revealed that OLs within MS lesions lacked an activating histone mark called H3K27ac, while expressing high levels of two other repressive histone marks H3K27me3 and H3K9me3 associated with silencing gene activity.
The research team scoured a library of hundreds of small molecules known to target enzymes that could modify gene expression and influence the silenced OLs. The team determined that the compound ESI1 (epigenetic-silencing-inhibitor-1) was nearly five times more powerful than any other compounds they considered.
The compound tripled the levels of the desired H3K27ac histone mark in OLs while sharply reducing levels of the two repressive histone marks. Additionally, the research reveals a new way in which ESI1 promotes the creation of special membrane-less regulatory hubs known as “biomolecular condensates” within the cell nucleus that control fat and cholesterol levels. These hubs act as central points to boost the production of essential fats and cholesterol needed to make myelin, a crucial component of nerve fibers.
In both aging mice and mice mimicking MS, the ESI1 treatment prompted myelin sheath production and improved lost neurological function. Testing included tracking gene activation, measuring the microscopic new myelin sheaths surrounding axons, and observing that treated mice were quicker at navigating a water maze.
Then the team tested the treatment on lab-grown human brain cells. The team used a type of brain organoid, myelin organoids, that is far more simplified than a full brain but still produces complex myelinating cells. When the organoids were exposed to ESI1, the treatment extended the myelin sheath of myelinating cells, the study reports.
MS is the most common and best known of several major neurodegenerative diseases. The new findings may spark a new approach to stopping the degenerative effects of these conditions, the senior author says.
Myelin regeneration treatment also could be helpful for people recovering from brain and spinal cord injuries.
But the most far-reaching implication of the study is the possibility of using ESI1, or similar compounds, to help slow or even reverse cognitive losses that often occur during aging. Many studies have shown that myelin loss plays a role in age-related loss of cognitive function, the author says.
However, more research is needed to determine whether human clinical trials can be launched to evaluate ESI1 as a potential treatment. For example, the effects of ESI1 may need to be modulated by adjusting the dose, treatment duration, or using “pulsed therapy” during specific time windows. More study also is needed to determine whether even more effective compounds than ESI1 might be designed from scratch.
“This study is a beginning,” the author says. “Prior to finding ESI1, most scientists believed that remyelination failure in MS was due to the stalled development of precursors. Now we show a proof of concept that reversing the silencing activity in OLs present in the damaged brain can