Lysosomal dysfunction strategy to treat glioblastoma
Glioblastoma is a type of brain cancer with a very poor prognosis of survival. Causes of glioblastoma are not known, and there is no method for preventing the cancer. Traditional treatment includes the drug temozolomide (TMZ). In many cases, TMZ kills glioblastoma cells, but a significant portion of patients show resistance to the drug.
Changes in the levels of metabolites — small molecules playing key roles in metabolic processes in living organisms — have been observed in TMZ-resistant glioblastoma cells, pointing to the importance of understanding and targeting metabolic pathways in the context of cancer therapy.
Now, the researchers have investigated the role of lysosomes — cellular organelles that break down biomolecules no longer needed — in metabolic processes linked to the development of glioblastoma. Based on their findings, they propose a targeted lysosomal dysfunction strategy for the treatment of glioblastoma.
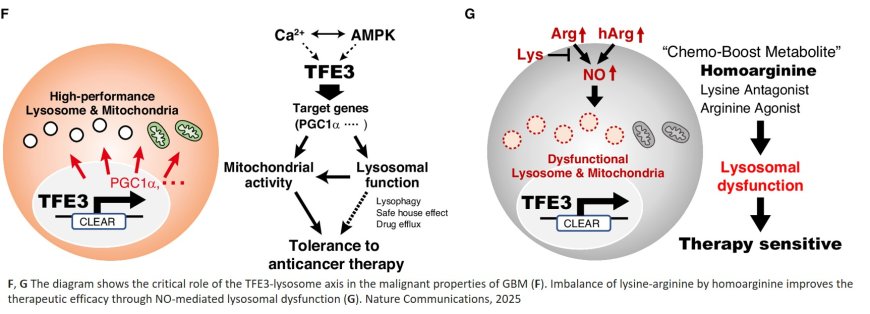
Lysosomes perform important biochemical functions. Apart from degrading ‘waste’ biopolymers, they are involved in cell signaling and energy flow and interact with other organelles. As such, they are also relevant for the development and growth of cancer. The researchers first checked whether lysosome activity is an indicator of glioblastoma progression. They looked at glioblastoma cell lines taken from patients and found a correlation between lysosomal activity and glioblastoma tumorigenesis and malignancy.
The researchers then investigated how lysosomal activity affects the therapeutic efficacy of TMZ. They found that when administering lysosomal inhibitors, the sensitivity of glioblastoma to TMZ increased, confirming the critical role played by lysosomes in glioblastoma. Further experiments pointed to a protein called transcription factor E3 (TFE3) as a crucial molecule for maintaining lysosomal function, and TMZ tolerance, in glioblastoma cells.
The scientists then studied which amino acids are essential for lysosomal activity in the context of glioblastoma progression. They discovered a correlation between lysine and glioblastoma malignancy. Lysine is not produced by the human body; it must be obtained through nutrition. The researchers therefore considered whether a lysine-restricted diet could be a therapeutic strategy for glioblastoma. They pointed out that, while lysine restriction might be a possible approach, it has limited practical use because of toxicity concerns – the human body needs lysine as a precursor to vital proteins.
Instead, the researchers worked out an alternative approach mimicking the effect of lysine restriction. Realizing that one of lysine’s functions is to antagonize the effect of arginine, which plays a role in the biosynthesis of nitric oxide, they tested the use of homoarginine, an antagonist of lysine — that is, inhibiting lysine’s function — to counteract lysine’s blocking of nitric oxide production from arginine, and induce lysosomal dysfunction. Experiments with mice showed that the combination of TMZ and homoarginine led to a significant suppression of glioblastoma cells, demonstrating its potential therapeutic value.
The work highlights the critical role of lysosomal function in glioblastoma pathogenesis, and how mimicking lysin restriction may play a role in anticancer strategies. Quoting the researchers: “… disrupting lysosomal function may provide a promising avenue for glioblastoma therapy.”
https://www.nature.com/articles/s41467-025-56946-z
https://sciencemission.com/Lysine-arginine-imbalance-to-treat-glioblastoma