Disrupting stress management in cancer cells
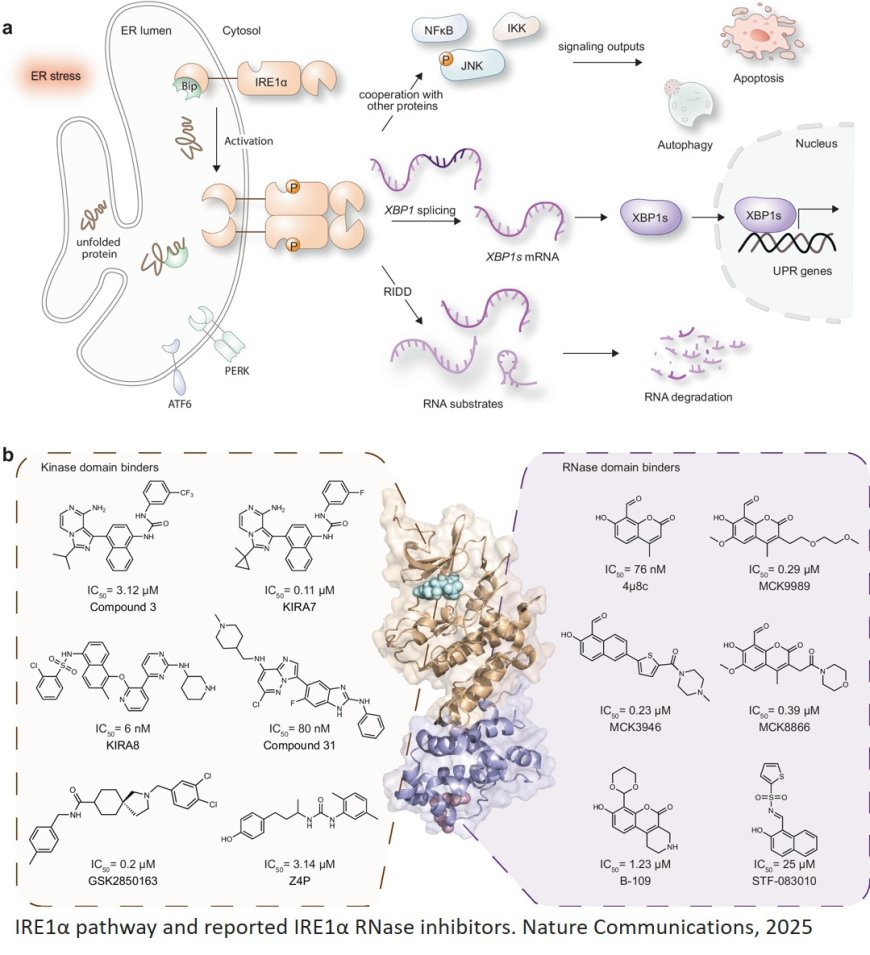
The laundry is still undone, the bicycle needs fixing, and bills haven’t been paid yet either. Unfinished tasks cause stress. The same principle applies to our cells. When too many proteins are incorrectly or even mis-folded, they cannot perform their functions and the cell becomes stressed. To cope with such stress, cells have evolved the unfolded protein response (UPR). Once activated by stress in the endoplasmic reticulum (ER) - the protein-producing organelle in the cell - a cascade of protective mechanisms is triggered to restore proper protein folding. One of the most important UPR transducers is IRE1, a protein embedded in the ER membrane. IRE1 is involved in a wide range of diseases, including immune, metabolic, and neuro-degenerative disorders as well as cancer and has thus become a relevant therapeutic target.
Tumors are often described as “wounds that never heal”. This is also due to the fact that cancer cells create a toxic environment, that is acidic, hypoxic and nutrient-deprived. Although this seems counter-productive, it is actually a clever strategy: the hostile-conditions activate evolutionary survival pathways, highjacked and repurposed to support tumor growth and survival.
“It is well known, that the activation of the UPR via IRE1 contributes to the development and progression of most cancers, such as leukemia, glioblastoma, myeloma, breast, and colon cancer. High IRE1 activity is also associated with increasingly poor prognosis”, says the author. Over the past decade, signalling proteins of the UPR have become attractive targets for the development of novel cancer therapies, and a growing toolbox of drug-like molecules is now available. However, many of these compounds have limitations.
The group has now developed a high-potency IRE1 inhibitor with a unique inhibition mode. First, the researchers developed a robust assay to evaluate the effect of potential IRE1 inhibitors. Using this assay, they screened a library of 10,000 chemically diverse compounds and identified indole-based scaffolds as particularly promising “hits” (such as IA107). Systematic structural optimisation yielded a lead compound that was then characterized biochemically, biophysically, and by its interaction with IRE1.
The researchers report a resolved co-crystal structure that reveals a unique inhibition mode of IA107 that allosterically inhibits IRE1α RNase activity via binding to the IRE1α kinase domain but without inhibiting the IRE1α dimerization.
They demonstrate that IA107 concentration-dependently inhibits the cellular ER stress-induced XBP1 mRNA splicing, and the ester-containing prodrug exhibits a ~ 50-fold increase in cellular activity.
Our understanding of the unfolded protein response has steadily evolved over the past few decades, and the first drug-like molecules targeting this process have shown promise in preclinical disease models. Yet many of the existing agents suffer from poor pharmacokinetics and cause significant side effects - particularly pancreatic toxicity. It is suspected that certain reactive moieties in these compounds interfere with cellular processes unrelated to IRE1 activity. Furthermore, some inhibitory mechanisms are not yet fully understood.
“Structural und functional studies such as ours, which clearly demonstrate the mechanism of action, are of great value and will accelerate the development of next-generation IRE1 inhibitors”, says the author. Such compounds could also serve as research tools to determine which approach to fighting cancer is most suitable in clinical practice and which diseases in humans can be treated most effectively by targeting the unfolded protein response.