Crystal structure of transthyretin implicated in amyloidosis
The tiny protein known as transthyretin can cause big problems in the body when it misfolds after secretion. While healthy transthyretin moves hormones through blood and spinal fluid, misfolded versions of the protein form dangerous clumps in the heart and along nerves—triggering a progressive and fatal disease known as transthyretin amyloidosis (ATTR). Up to a quarter of all men over the age of 80 have some degree of ATTR, which can cause shortness of breath, dizziness and tingling or loss of sensation in the extremities.
Now, scientists have uncovered new structures of transthyretin. Their findings, published in Nature Structural & Molecular Biology, show how the three-dimension asymmetry of the protein may contribute to its instability. This discovery could also contribute to the development of new drugs for ATTR.
“We’ve unveiled a molecular complexity that has been hidden from researchers for decades, which enables us to design better medicines to stabilize transthyretin,” says co-senior author.
“The new structures reveal differences in two thyroid hormone binding sites previously thought to be identical, and help explain why a drug binding to one site changes the ability of drugs to bind the opposing site,” adds another co-senior author of the study.
To determine the three-dimensional structure of small proteins such as transthyretin, researchers often turn to crystallography, in which proteins are forced into a large, repetitive crystal structure before being imaged. However, the structure of a crystallized protein doesn’t always reflect the conformation of individual free-floating proteins in the body.
Another method, cryo-electron microscopy (cryo-EM), flash-freezes proteins to catch them in their more natural structures. However, these frozen proteins are then suspended in a liquid and small proteins like transthyretin tend to get stuck at the air-liquid boundary rather than remaining fully submerged. This affects both the proteins’ structural stability and the ability to illuminate their detailed structure.
To overcome this challenge, the group developed a thin graphene-coated grid to which transthyretin molecules could naturally adhere. Then, they rapidly plunged this grid into liquid ethane to freeze the sample. This process trapped the transthyretin molecules in place on the graphene surface and preserved their natural conformations to mimic how they would appear when moving through the body’s blood or fluids.
“We built on the 2019 work from the Yan lab at Princeton when making our grids. Getting the surface chemistry right is crucial for this type of study. With small proteins like transthyretin, creating a high-quality sample is just the beginning; analyzing the data is also part of the challenge,” says the first author of the new paper.
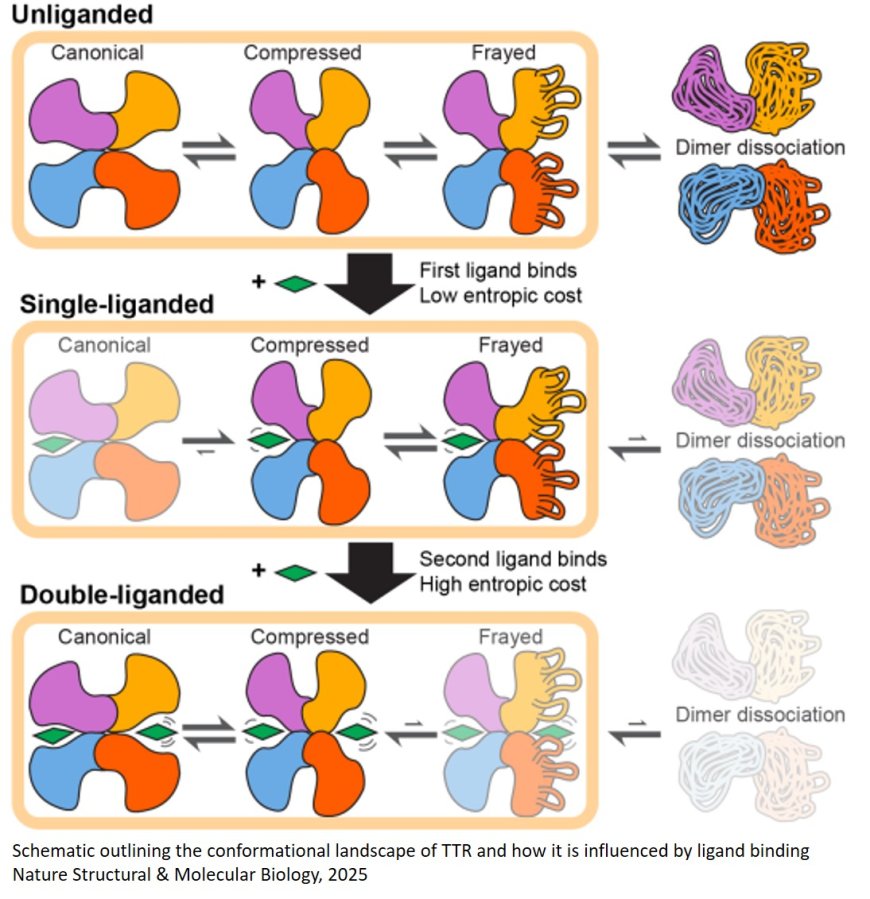
When the team tested the approach with transthyretin, they discovered that transthyretin forms asymmetric structures with two differently shaped binding pockets. Based on the more than 200 crystal structures that had been solved in the past, these binding sites had been assumed to be identical. The researchers showed that this variation was because the transthyretin complex was constantly wiggling between two different states—like a molecular version of “breathing,” according to the author. This asymmetry in the native structure of transthyretin also presents a hypothesis for how the process of dissociation and misfolding could occur then lead to the clumping of the protein and subsequent disease.
Attaching the ATTR drug tafamidis—to one or both of the transthyretin binding sites, they then found, stabilized the molecule and minimized this movement.
Now, the authors aim to study how this structure and its stabilization relate to ATTR, and how drugs targeting transthyretin could treat the disease. They also say that their graphene grid method could be used to determine the structures of other small and unstable proteins—including the amyloid-beta peptide that builds up in the brain in Alzheimer’s disease.
“The methodologies we’ve developed have opened new doors to avenues of treatment that could one day protect patients from not just TTR amyloidosis, but other amyloid diseases as well,” says the author.