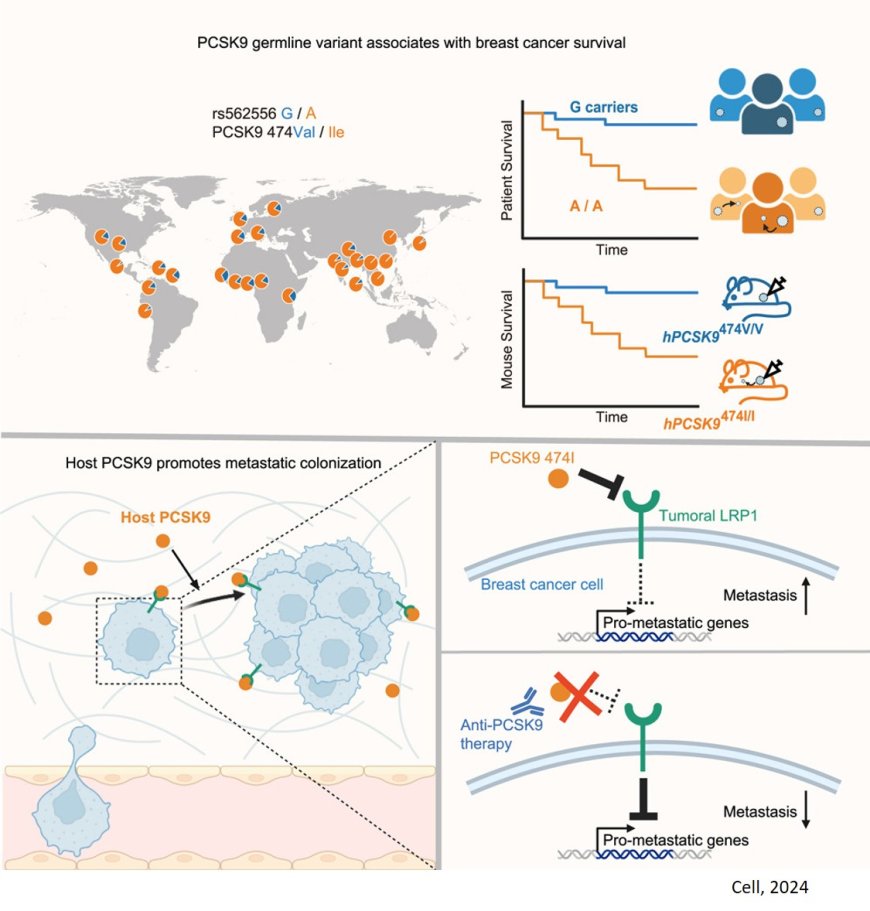
A genetic disposition increasing the risk of breast cancer metastasis
Ninety percent of cancer deaths are due to the spread of cancer, not complications from the original tumor. Thus, for over 50 years now, scientists have been on a quest to identify which malignant mutations within the tumor allow rogue cells to break away from the primary tumor and travel through the bloodstream and lymphatic system to metastasize throughout the body.
But new research suggests an alternative mechanism has been overlooked—elusive mutations driving metastasis may not be developing within the twisted DNA of tumors themselves, but within the patient’s regular, inherited DNA. The findings, published in Cell, provide the first evidence of genetic predisposition to breast cancer metastasis, with far-reaching clinical implications—including a potential therapy that could prevent it.
"Metastasis we believe is, at least in part, a hereditary disorder," says the senior author. "We have been so focused on the cancer cells, the 'seeds', that we've ignored the germline—'the soil'. It's now clear that focusing on the soil is critical."
Although scientists have rigorously investigated metastasis for decades, extensive genomic sequencing of metastatic tumors have come up empty. Patient tumors are full of mutated genes, but none have been shown to specifically drive metastasis. "So, we hypothesized instead that maybe the host's own genetics, not the tumor, is providing those mutations," the author says.
In fact, the researchers had previously demonstrated that various germline alleles of APOE can enhance or suppress melanoma metastasis. To find out whether breast cancer metastasis operates in a similar manner, the lab dove into human genetics with large patient cohorts from multiple countries.
A common variant of the PCSK9 gene immediately caught their attention. Present in the germlines of 70 percent of white women, this gene variant was associated with reduced breast cancer survival. And when the team engineered mice with the relevant variant form of human PCSK9, the rate of metastasis increased. The authors further validated these results with an analysis of a large Scandinavian cohort of early-stage breast cancer patients. Their findings were stark: patients with the PCSK9 variant faced a 22 percent risk of metastasis within 15 years, compared to a 2 percent risk among those without it.
"Our results emphasize the importance of international collaboration, which by definition involves researchers and patient cohorts from multiple countries," says the lead author on the paper. "Further, this demonstrates just how powerful human genetics has become. With new technologies that combine computational analysis with experimental models, we’re in a great era to answer difficult questions."
The study also sheds light on how the PCSK9 variant drives metastasis. By degrading the LRP1 receptor on cancer cells, the variant appears to unleash a cascade of gene activation ideal for metastatic initiation. Interestingly, the lab's prior work on melanoma found that the APOE alleles that promote or suppress metastasis also act on LRP1. "It's remarkable that, in two different cancers, the mechanism of metastasis converges on this one receptor," the author says. Future work from the lab will focus on LRP1's suspiciously consistent role in metastasis.
Despite the evidence now linking the PCSK9 variant to metastatic disease, the author clarifies that patients with this variant should not be alarmed. The data suggests that patients with the variant have a 22 percent risk of metastasis, as compared to a 2 percent risk without the variant. "The majority of patients with early-stage breast cancers harboring either variant will still never develop metastasis," the author says.
But the authors are optimistic about the possibility of reducing metastatic disease among the less fortunate 22 percent. The present study includes preliminary work suggesting that the PCSK9 variant can be suppressed with an antibody that blocks the activity of PCSK9 that is already approved for high cholesterol. "This is a safe and well-tolerated drug," the author says, while cautioning that clinical trials will be necessary to demonstrate its efficacy in cancer. "Our hope is that high-risk patients with this variant could one day be treated proactively, reducing their chances of metastasis by targeting the specific signals that elicit this outcome."