Antidepressant shows promise for treating brain tumors
Glioblastoma is a particularly aggressive brain tumor that at present is incurable. Cancer doctors can extend patients’ life expectancy through operations, radiation, chemotherapy or surgical interventions. Nevertheless, half of patients die within twelve months of diagnosis.
Drugs that are effective against brain tumors are difficult to find, as many cancer drugs often can’t cross the blood-brain barrier to reach the brain. This limits the choice of possible treatments. Neuro-oncologists have thus been searching intensively for some time to find better drugs that can reach the brain and eliminate the tumor.
Researchers have now found a substance that effectively combats glioblastomas, at least in the laboratory: an antidepressant called vortioxetine. Scientists know that this inexpensive drug, which has already been approved by agencies such as the FDA in the U.S. and Swissmedic, is capable of crossing the blood-brain barrier.
The lead author of the study found it using pharmacoscopy, a special screening platform that the researchers have developed over the past years. The study findings were recently published in the journal Nature Medicine.
With pharmacoscopy, the researchers can simultaneously test hundreds of active substances on living cells from human cancer tissue. Their study focused primarily on neuroactive substances that cross the blood-brain barrier, such as antidepressants, Parkinson’s medication and antipsychotics. In total, the research team tested up to 130 different agents on tumor tissue from 40 patients.
To determine which substances, have an effect on the cancer cells, the researchers used imaging techniques and computer analysis. Previously, the team had used the pharmacoscopy platform only to analyse blood cancer and derived treatment options from this. Glioblastomas are the first solid tumors that they have systematically investigated using this method with a view to use existing drugs for new purposes.
For the screening, the authors analysed fresh cancer tissue from patients who had recently undergone surgery. The researchers then processed this tissue in the laboratory and screened it on the pharmacoscopy platform. Two days later, the researchers obtained results showing which agents worked on the cancer cells and which did not.
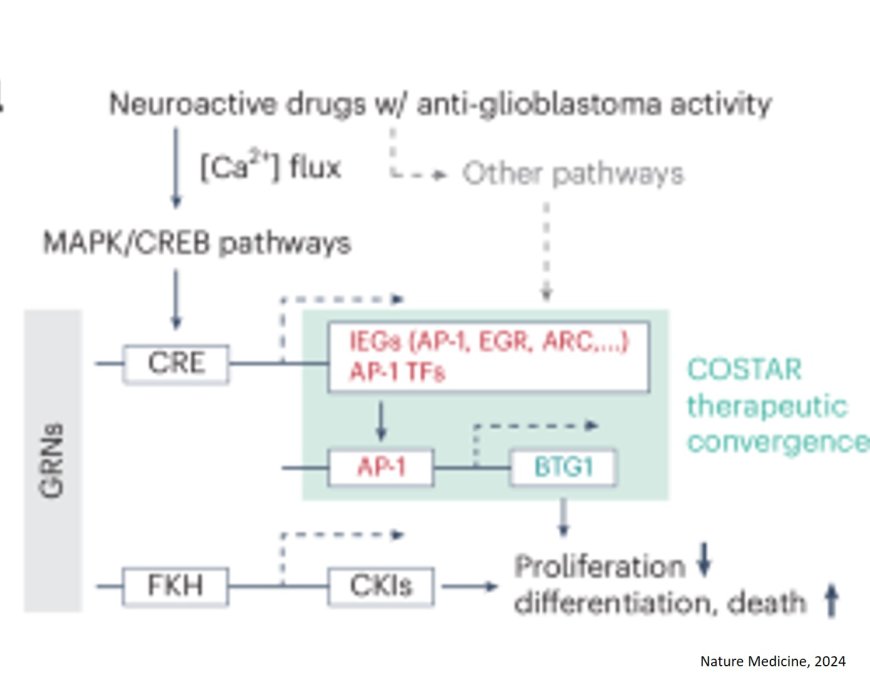
The results made it clear that some, but not all, of the antidepressants tested were unexpectedly effective against the tumur cells. These drugs worked particularly well when they quickly triggered a signalling cascade, which is important for neuronal progenitor cells, but also suppresses cell division. Vortioxetine proved to be the most effective antidepressant.
The researchers also used a computer model to test over a million substances for their effectiveness against glioblastomas. They discovered that the joint signalling cascade of neurons and cancer cells plays a decisive role and explains why some neuroactive drugs work while others don’t.
In the last step, researchers tested vortioxetine on mice with a glioblastoma. The drug also showed good efficacy in these trials, especially in combination with the current standard treatment.
The group of researchers is now preparing two clinical trials. In one, glioblastoma patients will be treated with vortioxetine in addition to standard treatment (surgery, chemotherapy, radiation). In the other, patients will receive a personalised drug selection, which the researchers will determine for each individual using the pharmacoscopy platform.
“The advantage of vortioxetine is that it is safe and very cost-effective,” says a coauthor of the study. “As the drug has already been approved, it doesn’t have to undergo a complex approval procedure and could soon supplement the standard therapy for this deadly brain tumor.”
However, the co-author cautions patients and their relatives against obtaining vortioxetine themselves and taking it without medical supervision. “We don’t yet know whether the drug works in humans and what dose is required to combat the tumor, which is why clinical trials are necessary. Self-medicating would be an incalculable risk.”