Role of physiological and pathological Aβ on synapses in human brain tissue
Scientists using living human brain tissue have shown for the first time how a toxic form of a protein linked to Alzheimer’s can stick to and damage the connections between brain cells.
Small pieces of healthy human brain tissue – collected during routine neurosurgery operations – were exposed to the protein, known as amyloid beta.
Unlike when subjected to a normal form of the protein, the brain tissue did not attempt to repair damage caused by the toxic form of amyloid beta, experts say.
The study also found that even small changes in the natural levels of amyloid beta – either increasing or decreasing – were enough to disrupt brain cells. This suggests the brain requires a finely tuned ‘sweet spot’ of the protein to function properly, experts say.
Researchers hope the discoveries will allow them to hone in on drugs that have the best chance of preventing the loss of synapses – connections which allow the flow of messages between brain cells and are vital to healthy brain function.
Alzheimer’s disease attacks synapses and their loss strongly predicts reduced memory and thinking abilities.
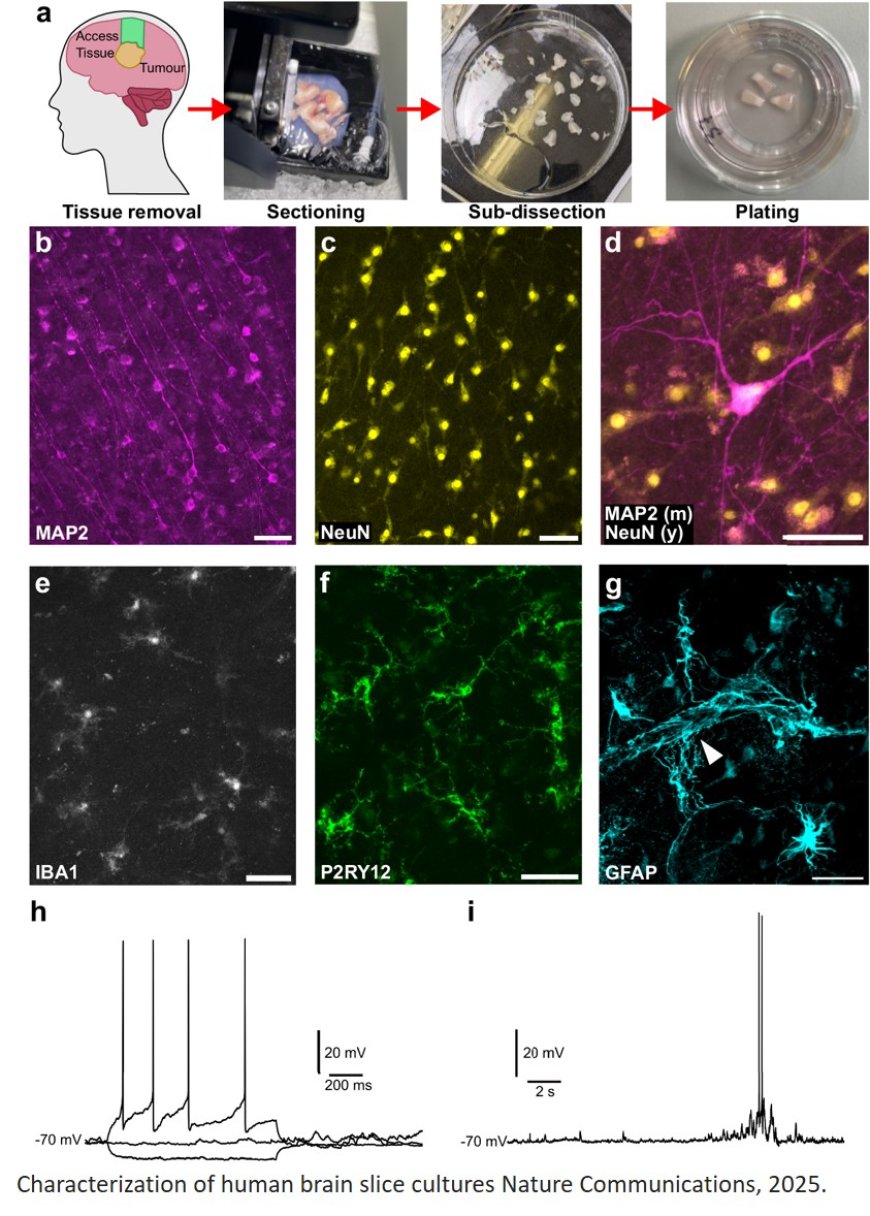
Central to the early success of the Project has been the pioneering method that keeps tiny fragments of human brain alive in laboratory dishes for several weeks after collection, with the patient’s permission.
This innovative approach offers a rare and powerful opportunity to investigate the early stages of Alzheimer’s disease in living human brain cells.
Using human brain tissue cultures, the researchers report that Aβ1-40 and tau release levels vary with donor age and brain region, respectively. Release of other biomarkers such as KLK-6, NCAM-1, and Neurogranin vary between brain region, while TDP-43 and NCAM-1 release is impacted by sex.
Researchers also discovered that brain slices taken from the temporal lobe, a region known to be affected early in Alzheimer’s, released higher levels of another key disease protein, called tau. This may help explain why this part of the brain is particularly vulnerable in the early stages of the condition, as increased tau release may enable faster spread of toxic forms of this protein between cells.
Pharmacological manipulation of Aβ in either direction results in a loss of synaptophysin puncta, with increased physiological Aβ triggering potentially compensatory synaptic transcript changes. In contrast, treatment with Aβ-containing Alzheimer’s disease brain extract results in post-synaptic Aβ uptake and pre-synaptic puncta loss without affecting synaptic transcripts.
In addition, a number of the samples were found to contain early indicators of Alzheimer’s, such as amyloid plaques and tau tangles, demonstrating the potential of this model to study the disease before symptoms appear.
Experts say this innovative approach will make it easier to test experimental drugs before they enter clinical trials, increasing the chance of finding drugs that work in the human brain.
https://www.nature.com/articles/s41467-025-58879-z
https://sciencemission.com/amyloid-%CE%B2-on-synapses-in-live-human-brain-slice-culture