Beta secretase
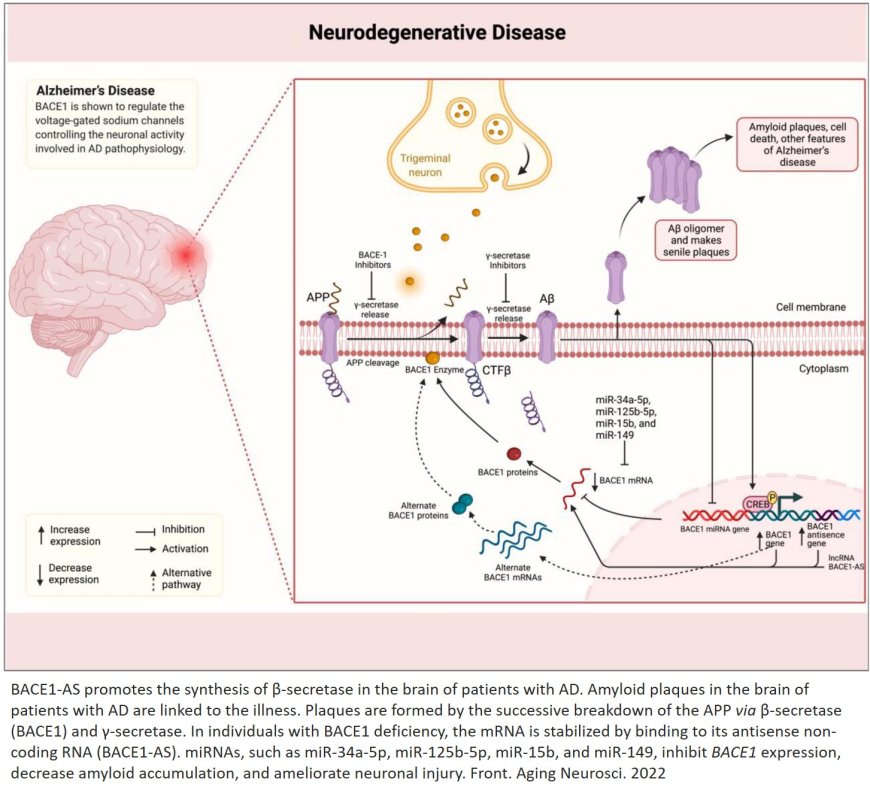
Beta-secretase (BACE1) is an enzyme that plays a crucial role in the pathogenesis of Alzheimer's disease (AD). Here's an overview of beta-secretase:
Structure and Function
1. Aspartic protease: BACE1 is a transmembrane aspartic protease that cleaves the amyloid precursor protein (APP) at the beta-site.
2. Zinc ion-dependent: BACE1 requires a zinc ion for catalytic activity.
3. Optimal pH: BACE1 has an optimal pH range of 4.0-4.5.
Substrates and Cleavage Sites
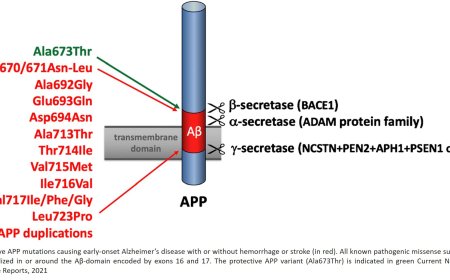
1. APP: BACE1 cleaves APP at the beta-site (between Met671 and Asp672), releasing the soluble APPβ (sAPPβ) fragment.
2. Other substrates: BACE1 also cleaves other substrates, including neuregulin-1, Jagged, and P-selectin glycoprotein ligand-1.
Role in Alzheimer's Disease
1. Aβ production: BACE1 initiates the amyloidogenic pathway by cleaving APP, leading to the production of amyloid-β (Aβ) peptides.
2. Aβ accumulation: Increased BACE1 activity contributes to Aβ accumulation, which is a hallmark of Alzheimer's disease.
3. Neurotoxicity: BACE1-mediated Aβ production can lead to neurotoxicity and contribute to neurodegeneration.
Inhibitors and Therapeutic Potential
1. BACE1 inhibitors: Several BACE1 inhibitors have been developed, including verubecestat, lanabecestat, and umibecestat.
2. Clinical trials: BACE1 inhibitors have been tested in clinical trials for Alzheimer's disease, but results have been mixed.
3. Challenges: Developing effective BACE1 inhibitors has been challenging due to the enzyme's complex structure and the need to balance inhibition with potential side effects.
Regulation of BACE1 Activity
1. Transcriptional regulation: BACE1 expression is regulated by transcription factors, including SP1 and NF-κB.
2. Post-translational modifications: BACE1 activity can be regulated by post-translational modifications, such as phosphorylation and ubiquitination.
3. Protein-protein interactions: BACE1 interacts with other proteins, including APP, to regulate its activity.
https://www.frontiersin.org/journals/aging-neuroscience/articles/10.3389/fnagi.2022.853180/full