Cancer-associated gene can mimic muscle growth from exercise
Researchers have long known that there is a relationship between the cancer-associated gene MYC (pronounced “Mick”) and exercise adaptation. When human muscles are exercised, MYC is found to increase transiently in abundance over 24 hours. But as we age, the MYC response to exercise is blunted, perhaps explaining a reduced ability to recover from exercise and maintain or gain muscle.
Knowing the precise mechanisms by which MYC drives muscle growth could prove instrumental in creating therapies that reduce muscle loss from aging, potentially improving independence, mobility and health.
New research published in EMBO Reports now adds an important dimension to our understanding of the role of MYC in skeletal muscle.
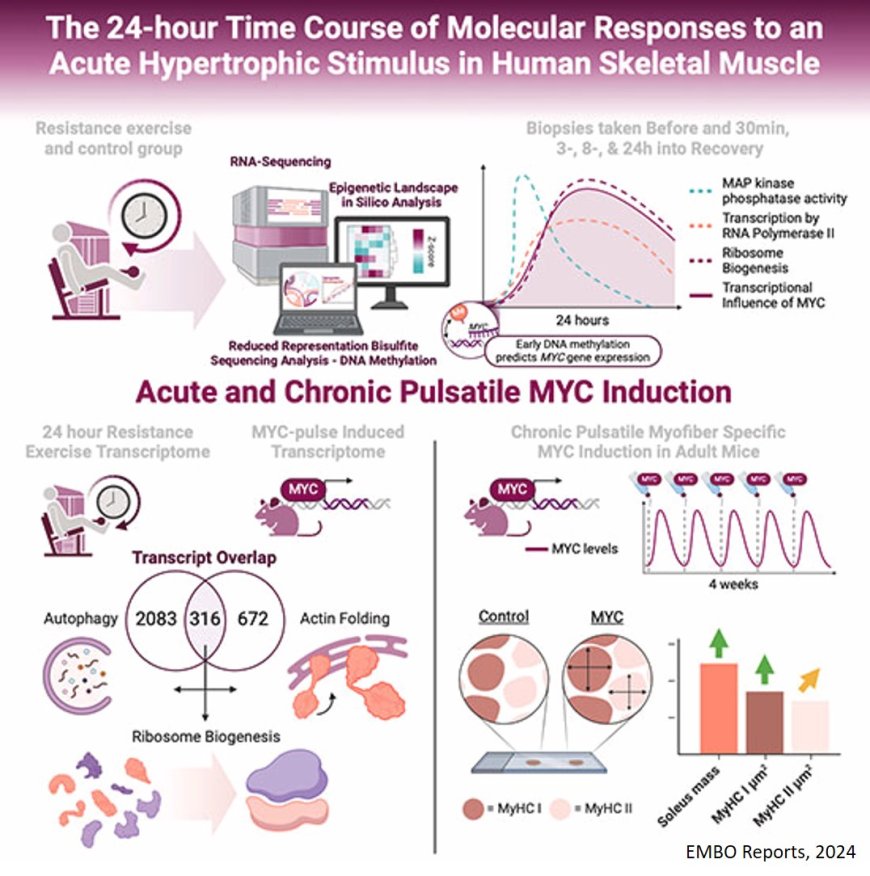
Given so many contributors, the paper is rich with data but essentially boils down to two parts. The first is a 24-hour chronicle of the molecular landscape of the human muscles following resistance exercise. The second half examines the use of mouse models to determine if controlled doses, or pulses, of MYC within skeletal muscles would be enough to stimulate muscle growth independent of actual exercise. The short answer: yes.
Co-first author noted that most studies tend to look at the molecular landscape of the human body by taking biopsies prior to exercise and then a few hours later. But by taking multiple biopsies over a period of 24 hours, which the team in oversaw, the researchers were able to get a more complete profile of how the body adapts to exercise over time and what genes are most important in that process.
“We show that the peak of responsiveness and where most things were happening was actually eight hours after exercise,” the author explained and added that they found that three hours after exercising, MYC ranked as the third most important molecule. “And then at eight and 24 hours, it was the most influential. So it was really important to get those time points and to map out the body’s response to acute exercise.”
Once the researchers had a clearer understanding of what was happening molecularly in human muscles over time, they wanted to isolate MYC and see if it alone was enough to facilitate muscle growth. This was done by genetically controlling the levels of MYC in their skeletal muscles using a specialized mouse model. The mice weren’t given an exercise wheel, which would naturally promote muscle growth, but were otherwise allowed to move around normally.
Samples were then taken from the soleus muscles of their lower legs, which are utilized in basic activities like standing or walking around. Analysis confirmed that MYC alone led to increased muscles mass and fiber size in the soleus in comparison to genetically identical mice that did not have MYC pulses but otherwise lived under identical circumstances. Thus, the team was able to effectively “mimic” the exercise response without exercise.
These findings further the argument that MYC is a key player in muscle growth from resistance training. Even so, MYC is not likely to be the basis of a new therapy for sarcopenia or a performance-enhancing drug. MYC regulates roughly 15 percent of the estimated 20,000 genes in the human body, meaning it could have unpredictable downstream effects involving thousands of genes. It is also a potent oncogene, meaning the very growth it promotes in skeletal muscle could stimulate cellular proliferation if overexpressed in organs like the liver, resulting in tumors. Administering MYC alone could have unintended and deadly side effects.
The senior and corresponding author on the paper commented that “it’s interesting that one of the things that is known to cause cancer also regulates the muscle growth response to exercise. This suggests shared regulation and that ‘growth is growth.’”
The author added, “The take-home isn’t necessarily that we need to induce MYC in muscle to mimic exercise, but that we can harness the knowledge of what this oncogene affects in muscle and then try to design therapies and interventions for atrophy and enhancing muscle adaptability that activate those positive downstream effects of MYC without evoking the possibility of oncogenesis.”
In addition to being an oncogene, MYC is also one of the four Yamanaka factors, which are four protein transcription factors that can revert highly specified cells (such as a skin cell) back to a stem cell, which is a younger and more adaptable state. In the correct dosages, inducing the Yamanaka factors throughout the body in rodents can ameliorate the hallmarks of aging by mimicking the adaptability that is common to more youthful cells.
Of the four factors, only MYC is induced by exercising skeletal muscle. These findings provide further motivation for the researchers to understand what MYC is doing in muscle from an aging context with exercise.
Moving forward, Jones will continue to dig deeper into the mysteries of MYC as the focus of his dissertation. “I’m super passionate about it,” the author said. “I wake up every day thinking about this project. I love working on this project, and I think MYC is one of the most heavily influential molecules in muscle tissue… but there is still so much we don’t know.”
https://www.embopress.org/doi/full/10.1038/s44319-024-00299-z