DNA damage in chronic traumatic encephalopathy (CTE)
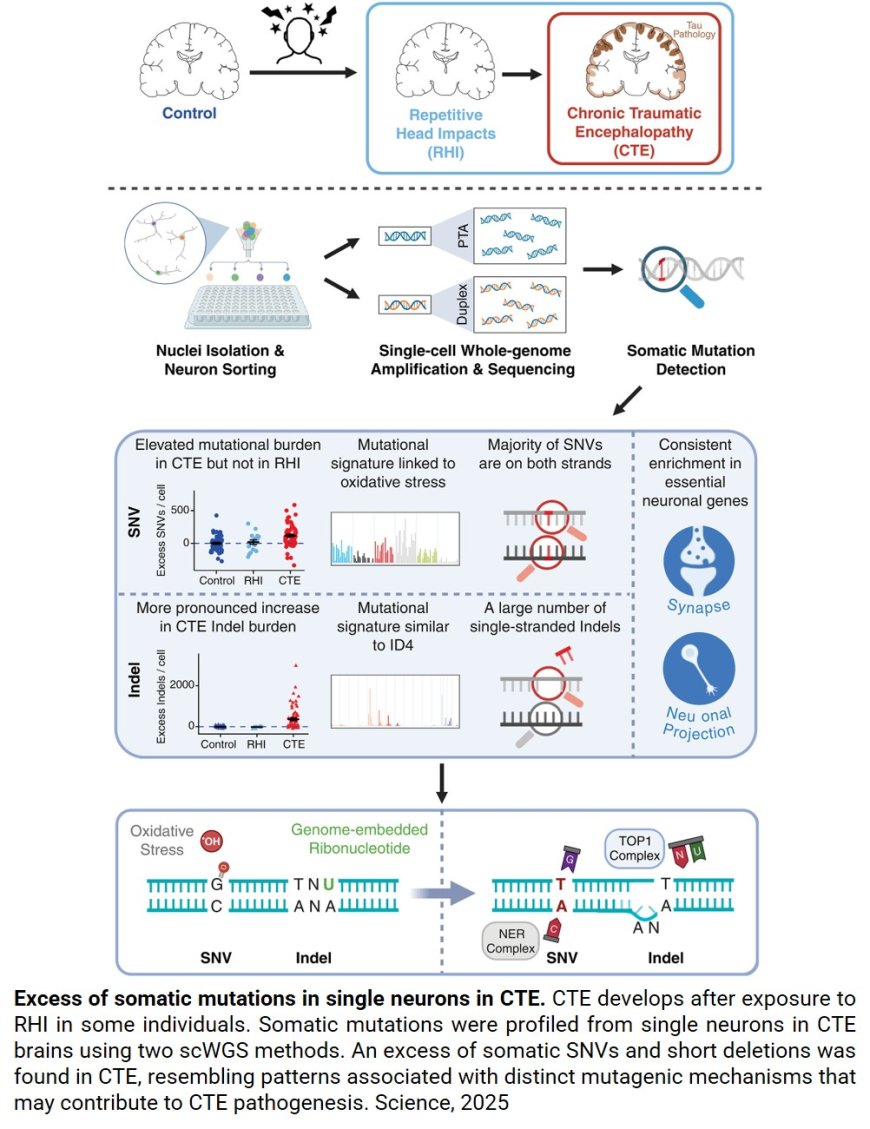
Chronic traumatic encephalopathy (CTE) – most often found in athletes playing contact sports – is known to share similarities with Alzheimer’s disease (AD), namely the buildup of a protein called tau in the brain. New research published in Science finds even more commonalities between the two at the genetic level, showing CTE (like AD) is linked to damage to the genome and not just caused by repeated head impact (RHI).
The research team used single-cell genomic sequencing to identify somatic genetic mutations (changes in DNA that occurs after conception and are not hereditary). They analyzed hundreds of neurons from the prefrontal cortex of 15 individuals diagnosed with CTE postmortem and 4 individuals with RHI but without CTE and compared their findings with 19 neurotypical controls and 7 individuals with AD.
The team found neurons from brain samples with CTE had specific abnormal patterns of somatic genome damage that closely resemble those seen in AD. Notably, brain samples that displayed signs of RHI without CTE didn’t have these changes. They also observed that those with CTE showed signs of damage equivalent to 100+ years of excess aging.
“Our results suggest that CTE develops through some process in addition to head trauma,” said co-corresponding author of the study. “We suspect it involves immune activation in a way similar to Alzheimer’s disease, happening years after trauma.”
RHI most often occurs during contact sports such as American football, hockey, or rugby or during military service. CTE has been found postmortem in the brains of teenagers and young adults playing amateur sports, as well as in older professional athletes.
Recent research from another co-corresponding author found RHI causes brain damage in young people even before CTE. Their study, published in Nature, suggested that RHI-related brain injury occurs before the onset of tau deposition as CTE. This new research published adds on to this growing evidence base.
“One of the most significant aspects of our work is the introduction of a new, single-cell genome approach to CTE,” said another co-corresponding author. “Our study provides further evidence that CTE is a bona fide neurodegenerative disease defined by its unique neuropathological features.”
Given the shared mechanisms found between CTE and AD, there could be promise in identifying shared novel targets for these two neurodegenerative diseases.