Glycation-lowering compounds curb hunger, lower insulin resistance and extend lifespan
The same chemical reaction that makes a piece of freshly toasted bread delicious also happens in our bodies, with far less appetizing consequences. We’re talking about the Maillard reaction, where sugars react with protein to form brown, sticky compounds in a process called glycation. Glycation is increasingly suspected to be a hidden driver of obesity, diabetes and accelerated aging. Researchers have found a way to tame it in mice by feeding them a combination of glycation-lowering compounds.
In a study published in Cell Reports, scientists show that a combination of nicotinamide, a-lipoic acid, thiamine, pyridoxamine and piperine (dubbed Gly-Low) curbed hunger, lowered insulin resistance and extended lifespan in mice by reducing advanced glycation end-products (AGEs), sticky plaque-like molecules which are a byproduct of glycation.
Senior scientist says AGEs gum up the machinery of life. “Once formed, AGEs are hard to remove,” the author says, comparing them to rust that corrodes metal. “As we age our defenses against glycation weaken. Scientists have long suspected that AGEs accelerate many age-related diseases. Our results provide a strong proof-of-concept that glycation isn’t just a bystander in aging; it may be a modifiable target to help people live healthier, longer lives.”
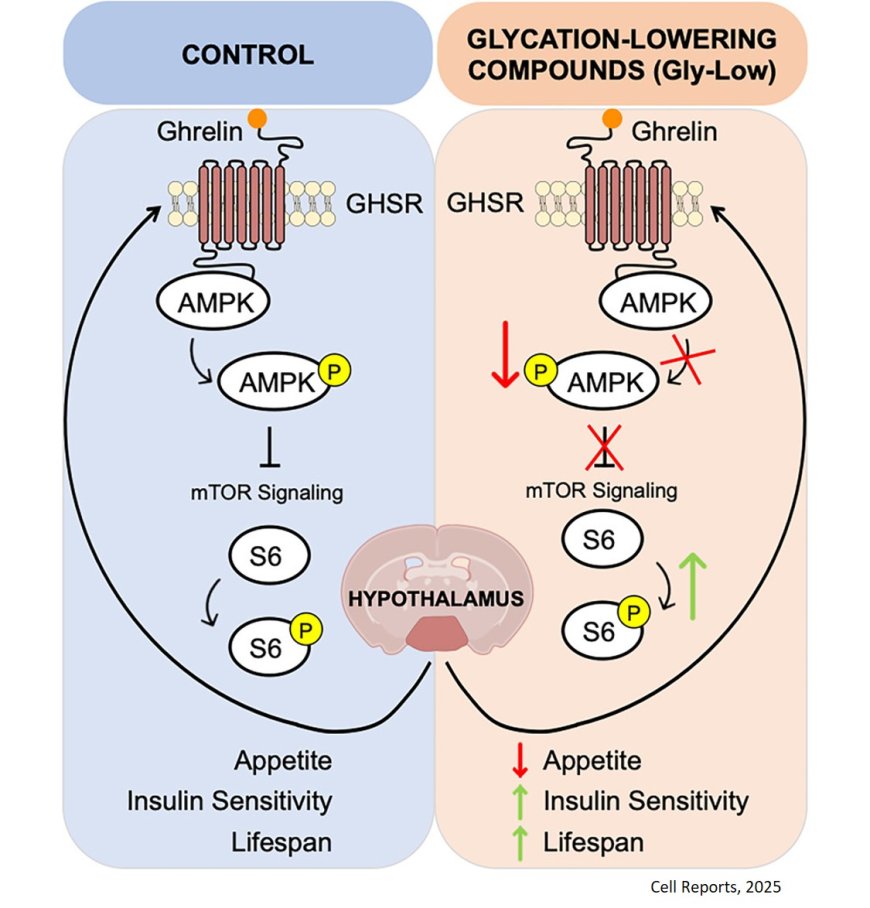
The authors delved into the mechanism by which Gly-Low was impacting normal physiology and feeding behavior in both diabetic and non-diabetic mice. They discovered that the compounds impacted hypothalamic ghrelin signaling (ghrelin is often called the “hunger hormone”), leading to a decrease in feeding behavior and thereby reducing glycation stress and combatting obesity.
They also analyzed the hypothalamic transcriptome of Gly-Low treated mice and found that the combination of compounds changed signaling through the cellular energy sensor AMPK activating the mTOR/S6 pathway in the hypothalamus. “Gly-Low shifted the balance away from ‘feed me’ signals toward satiety,” said the author. “The effect was profound; the mice voluntarily ate less food while maintaining muscle mass. Our data suggest that rather than simple food aversion, the biology of hunger was being rewired.”
The study also showed that treated mice had lower fasting and random blood glucose, better insulin sensitivity and reduced fat accumulation in their livers.
The senior author thinks the most exciting result of the work may be Gly-Low’s efficacy as a late-life intervention. In one set of experiments, treating the mice at 24 months, which is roughly equivalent to a 70-year-old human. The author says the compounds helped the older mice control blood sugar surges and the aging animals had enhanced motor coordination and a longer life; Gly-Low extended their remaining lifespan by nearly 60 percent.
“What’s remarkable here is that caloric restriction, the gold-standard longevity intervention, usually fails to extend lifespan if started late in life. Gly-Low, by contrast, succeeded,” says the author. “That suggests its effects go beyond just eating less—it is reversing some of the molecular hallmarks of hypothalamic aging itself,” he says, adding that in transcriptomic analyses, genes that normally change with age were shifted back toward youthful patterns in Gly-Low treated mice.
The author says that each of the compounds that go into the Gly-Low “cocktail” are known to influence metabolism or glycation in some way. “Individually some of these compounds can modestly affect appetite or glucose balance. But together they act synergistically to dramatically reduce glycation stress and alter feeding behavior.”
Playing off the toast analogy, the author says that the older we get the more “browned” our biology becomes. “Mice are not humans, and we need rigorous human trials before any clinical use,” the author says. “But the implications are broad. Many age-related diseases, from Alzheimer’s to kidney disease, have high levels of AGEs. If glycation can be safely targeted, it may open new doors to treating a spectrum of conditions at once.”
https://www.cell.com/cell-reports/fulltext/S2211-1247(25)01193-3