Mechanism of endothelial insulin resistance
The hormone adrenomedullin disrupts insulin signaling in blood vessel cells, contributing to systemic insulin resistance in obesity-associated type 2 diabetes, according to a new study.
Blocking adrenomedullin’s effects restores insulin function and improves glucose control in a mouse model, suggesting a potential new target for treating obesity-related metabolic disease.
Diabetes is a leading global cause of illness, mortality, and healthcare expenditures, with most cases stemming from obesity-induced insulin resistance and type 2 diabetes mellitus. Insulin resistance primarily affects key metabolic cells, including those in skeletal muscle, adipose tissue, and the liver.
Endothelial cells within blood vessels also express insulin receptors, and endothelial insulin signaling is thought to play a crucial role in metabolic regulation.
Previous research has suggested that endothelial insulin resistance may contribute to the systemic insulin resistance of type 2 diabetes. However, the precise mechanisms underlying endothelial insulin resistance remain unclear, and its direct role in type 2 diabetes has yet to be definitively established.
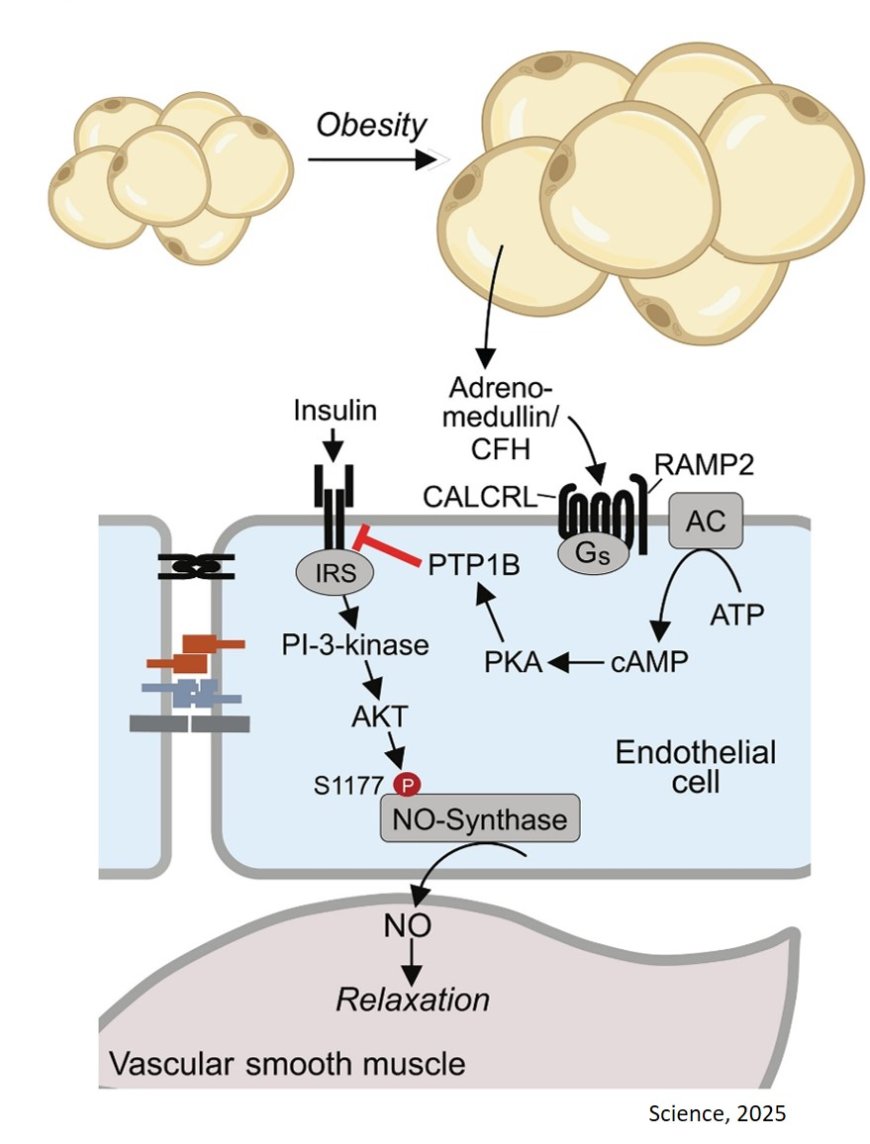
The researchers found that plasma levels of the hormone adrenomedullin and complement factor H (CFH) – a protein that enhances adrenomedullin’s effects – were elevated in the blood of obese mice and humans.
According to the authors findings, in human endothelial cells, adrenomedullin inhibits insulin signaling by protein-tyrosine phosphatase 1B–mediated dephosphorylation of the insulin receptor, thereby deactivating the insulin receptor, suggesting that higher levels of adrenomedullin and CFH in obesity contribute to insulin resistance in blood vessels.
Highlighting these findings, the authors demonstrate that treating lean mice with adrenomedullin caused insulin resistance and poor glucose control, mimicking obesity. This effect was eliminated in mice lacking adrenomedullin receptors in their blood vessels, confirming that the hormone acts through these receptors.
In obese mice lacking the endothelial adrenomedullin receptor, insulin-induced endothelial nitric oxide–synthase activation and skeletal muscle perfusion were increased.
Furthermore, blocking adrenomedullin signaling improved insulin function in blood vessels, enhanced muscle blood flow, and prevented insulin resistance in obese mice, highlighting its central role in obesity-related metabolic dysfunction.