Ketone metabolites from shunt pathway prevents obesity
The ketogenic “keto” diet and intermittent fasting have surged in popularity, embraced by everyone from weekend warriors to endurance athletes. These trends promise to harness the power of ketosis — a metabolic state where the body burns fat for energy instead of carbohydrates. Advocates tout its benefits, from weight loss to neuroprotection.
A collaborative research team is now tackling the unanswered questions surrounding ketosis.
Rather than adding to the growing, and often confusing, literature on the effects of ketogenic diets, the team are focused on the underlying chemistry of ketones themselves.
Their discovery — a previously unknown metabolic pathway and a family of “keto” metabolites — could rewrite our understanding of how ketosis influences metabolism, including in the brain.
“It turns out ketosis is not a monolithic state," said the author. "There’s a lot more complexity and nuance in how the body processes ketone molecules, and this could explain some of its more intriguing effects."
The research — published in the journal Cell.
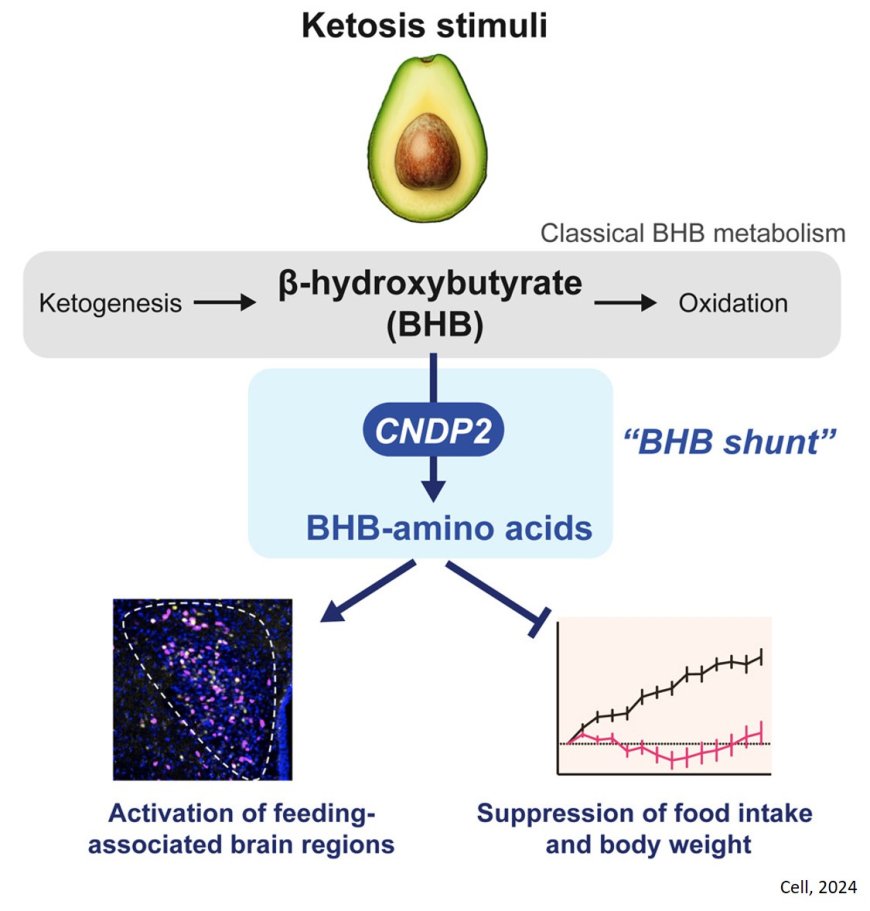
When deprived of glucose — its primary energy source — the body shifts gears, breaking down fat to produce ketones as an alternative fuel. Central to this process is beta-hydroxybutyrate (BHB), the most abundant ketone body.
Until now, scientists believed ketosis followed two main biochemical pathways: ketogenesis, which produces BHB in the liver, and ketolysis (or ketone oxidation) which consumes BHB for energy throughout the body. These pathways were thought to tell the whole story.
The team weren’t so sure. They decided to take another look at what ketones, particularly BHB, were doing in the body. Rather than diving into the already contentious literature on the ketogenic diet’s downstream effects — such as its potential benefits for cognition or metabolic health — they decided to take a step back.
“Let’s just step away from all the purported effects and focus on the chemistry of these metabolites,” the author explained. “Where do they come from? Where do they go?”
In a series of experiments on mice and humans, the researchers manipulated the availability of BHB to explore how it influences metabolism and energy balance. What they found was a previously unknown metabolic "shunt pathway," where enzymes attach BHB to amino acids, producing a family of compounds they dubbed BHB-amino acids.
"If pathways are like the highway system, shunts are the off-ramps," the author explained. "What we're saying is, this is not the main pathway that's directing traffic, but it gets you somewhere very interesting and unusual off the main road."
The discovery of this ketone shunt suggests that ketones have additional, previously unrecognized roles in the body’s metabolic landscape. The critical question remained: Are they inert byproducts, or do they actively influence the body’s response to ketosis?
To answer these questions, the authors zeroed in on the brain — a focus driven by a well-documented phenomenon: when people are in ketosis, their hunger often decreases.
“When I’m fasting or losing weight, I don’t feel as hungry,” said the author. “That’s a well-established aspect of ketosis, tied to the neurobiology of feeding and energy balance.”
Further, the team noticed that the metabolites they were studying chemically resembled another molecule that is known to regulate hunger and appetite: Lac-Phe. Lac-Phe is produced in the body after sprint exercise, and functions to reduce appetite. This chemical resemblance guided their investigation, raising the question: Could these ketone metabolites play an active role in appetite suppression and weight regulation under ketosis conditions?
The researchers found that BHB-amino acids suppress feeding behaviors and promote weight loss, revealing a potent link between ketosis and energy regulation. “This third, shunt pathway turns out to be important for the regulation of appetite and ketosis-associated weight loss,” said the author.
By uncovering this previously unknown pathway, the researchers have created an opportunity to revisit longstanding questions about the mechanisms behind the ketogenic diet’s purported benefits.
Until now, “our basic understanding [of ketosis] was actually incomplete,” said the author. “Now, we can revisit all these phenomena through a new lens.”
For instance, while it’s well established that the ketogenic diet is uniquely effective in controlling seizures in children with drug-resistant epilepsy, it remains unclear whether other benefits, such as improved cognition or metabolic health, are real — and, if so, how they work. The identification of these metabolites offers a new framework for investigating these effects systematically.
In fact, the authors are already revisiting epilepsy. The authors are investigating whether the newly identified shunt pathway and its metabolites play a role in seizure control. If so, this could open the door to novel treatments that replicate the benefits of ketosis without requiring a strict dietary regimen.
As the team continues to probe the fundamental biology of ketosis, their work could pave the way for a deeper understanding of its therapeutic potential — not just for epilepsy but for a range of metabolic and neurological conditions.
“Now that we have a better understanding of these pathways, we can ask much better questions about how and why these products might work — and what risks or limitations they might carry,” said the author.