New insights into ALS-linked protein
Using advanced techniques in biophysical chemistry, a research team has achieved unprecedented views of a protein that may play a pivotal role in some cases of amyotrophic lateral sclerosis (ALS) and the related disorder frontotemporal dementia (FTD). Their work could open doors to new approaches for treatment and prevention.
The team described the advance in the journal Molecular Cell.
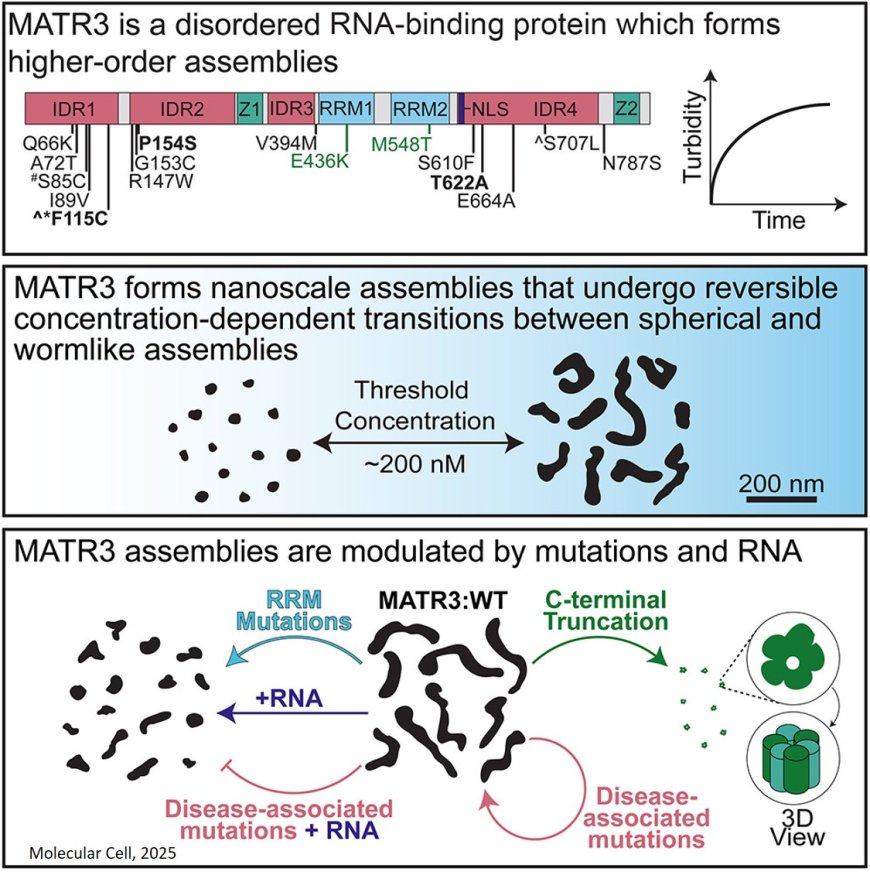
The team focused on Matrin-3, a little-studied protein with potentially broad health implications. Normally, the protein helps regulate nucleic acids, cell survival, differentiation and gene expression. Mutations in Matrin-3 can cause it to misfold, taking on abnormal functions.
Over time, misfolded proteins can disrupt nerve function and contribute to neurodegenerative diseases such as ALS, also known as Lou Gehrig’s disease, and FTD, a type of dementia caused by damage to the neurons in the brain’s frontal and temporal lobes.
To better understand the disease process and explore potential treatments, the team aimed to produce a clear picture of Matrin-3 — a challenge that took years to overcome.
One major hurdle was finding a way to chemically separate the protein from the rest of the cell. After much trial and error, the authors were able to isolate pure samples of Matrin-3.
“Purifying Matrin-3 was a very formative portion of my graduate studies,” the author said. “I knew it was going to be challenging, and I ended up spending over two years troubleshooting this process. I was about ready to give up when we finally found an approach that worked.”
The team then used advanced electron microscopy to characterize the shapes of the protein assemblies. “We found tiny spheres that seemed to transform naturally into worm-like shapes,” another author said. “We’ve never seen these types of shapes before, so that was our first hint that something unusual was happening.”
The team determined that the transition from spheres to worms likely occurs through a process called microphase separation.
When non-mutated Matrin-3 protein was mixed with RNA, the worm-like assemblies significantly shortened. “Matrin-3 is an RNA-binding protein, so it was important for us to study that interaction,” the author said.
Mutated versions of the protein found in disease also formed spheres and worms, but their length remained largely unchanged when exposed to RNA. “The mutations associated with ALS seem to be making these worm-shaped proteins more resistant to change,” the author said. “This may be a part of the disease process.”
With a reliable method to purify Matrin-3, as well as new ways to image the protein in living cells, the lab plans to continue experiments to better understand the protein’s role in health and disease. It is also likely that microphase separation is a widespread phenomenon that has not been well-studied due to the very small size of these assemblies, so they are also studying this idea more broadly.
“Macy’s work to purify the protein and figure out how to experimentally study these extremely small assemblies was a major advance for our lab and for studying the root causes of ALS,” the author said. “We’re excited to take the next steps.”
https://www.cell.com/molecular-cell/fulltext/S1097-2765(25)00740-3
https://sciencemission.com/Matrin-3-forms-spherical-and-wormlike-assemblies