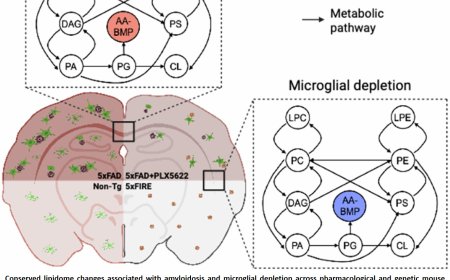
A dendritic nanotubes for intercellular communication in brain
Scientists say they have shown how mammalian brains create networks of tubes that transport toxins in and out of brain cells, similar to how pneumatic tubes swoosh items back and forth in factories and stores.
The experiments, conducted with genetically engineered mice and specialized imaging technology and published in the journal Science. These findings may advance understanding of the processes that lead to Alzheimer’s disease and other neurodegenerative conditions and pave the way for potential new therapies, the scientists say.
Specifically, in their experiments, the scientists say they observed nanotubes forming primarily to rid neurons of toxic small molecules, including amyloid-beta, a protein that lumps together and forms sticky deposits that are a hallmark of Alzheimer's disease.
"Cells have to get rid of toxic molecules, and by producing a nanotube, they can then transmit this toxic molecule to a neighbor cell," says corresponding author. "Unfortunately, this also results in spreading harmful proteins to other areas of the brain."
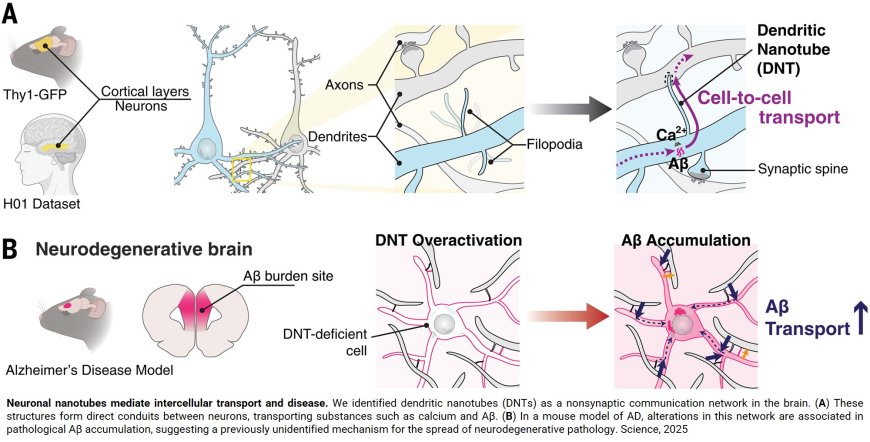
Using high-powered microscopes and live cell imaging of neurons, the scientists say they observed how brain cells produce a layer of long, finger-like protrusions between brain cell dendrites, the arm-like extensions that branch off cells. The scientists say these protrusions, so-called "dendritic nanotubes," ferry small, harmful molecules from brain cell to brain cell.
“The long and thin column-like structures of these dendritic nanotubes help transfer information quickly from neuron to neuron,” says the author. “These nanotubes can transport calcium, ions or toxic molecules, and are ideal for sending information to cells that are far away.”
Computational models of the process the researchers documented in the new experiments mimic the process of “early amyloidosis,” the team reports, and “uncover a nanotubular connectivity layer in the brain” that goes beyond usual communication between brain cells.
The author says these findings could help researchers better understand how to develop treatments for neurodegenerative conditions including Alzheimer's disease.
In their experiments, the authors took small tissue samples from the brains of normal lab mice and used high-powered microscopes to characterize the function and structure of nanotubes within neurons. New microscope technology enabled the scientists to see the nanotube structures in fine detail and observe how they transferred materials between brain cells.
The scientists then compared brain tissue samples from the normal mice to brain tissue samples from mice genetically engineered to develop the hallmark amyloid buildup of Alzheimer's disease.
The researchers say the mice with Alzheimer's disease had an increased number of nanotubes in their brains at three months old, when the mice were symptom-free, as compared with normal mice of the same age. At six months of age, the number of nanotubes in normal mice and those with Alzheimer's disease began to equalize.
By taking a closer look at human neurons (sampled with permission from a publicly available electron microscopy database), the scientists identified nanotubes with similar morphology forming between neurons in the same way that the laboratory mice developed them.
In future experiments, the team will focus on whether larger-scale nanotube networks exist in cell types other than neurons in the brain. Eventually, he intends to design an experiment in which researchers create a nanotube to see how it affects the state of cells.
With such knowledge, the author says, there’s the possibility of one day dialing up or down nanotube production to protect the brain.
“When designing a potential treatment based on this work, we can target how nanotubes are produced — by either increasing or decreasing their formation — according to the stage of the disease,” the author says.