Mechanism to reactivate dormant neural stem cells
An international team of neuroscientists have uncovered a mechanism that controls the reactivation of neural stem cells, which are crucial for repairing and regenerating brain cells. The research, published in Nature Communications, offers exciting potential for advancing our understanding and treatment of common neurodegenerative diseases like Alzheimer’s and Parkinson’s disease.
Neural stem cells are the source of the brain’s primary functional cells. After the initial development of the brain, neural stem cells typically enter a dormant state, conserving energy and resources. They re-awaken only when the brain needs them, such as after an injury or with physical exercise. However, with age, fewer neural stem cells can be roused from their dormant state, leading to various neurological conditions. Understanding how this reactivation is regulated is essential for developing treatments for various neurological conditions.
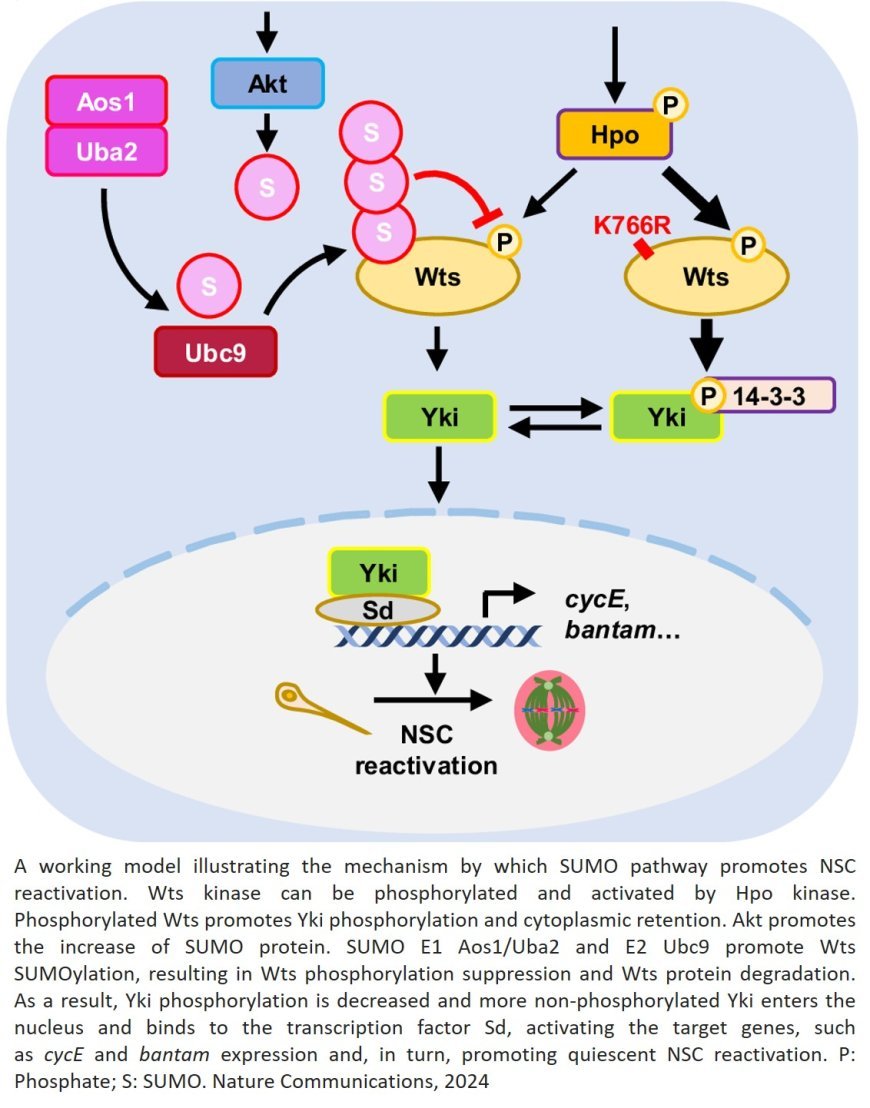
In this study, the team discovered that a specific group of proteins play an essential role in “waking up” dormant neural stem cells through a process called SUMOylation.
In SUMOylation, a small protein named SUMO (small ubiquitin-like modifier) tags target proteins inside a cell to influence their activity and/or function. These SUMO-tagged proteins, the researchers found, trigger the reactivation of neural stem cells, allowing them to contribute to brain development and repair. Conversely, without SUMO proteins present, the fruit flies produced a microcephaly-like phenotype. This is the first study to pinpoint the SUMO protein family’s exact role in the reactivation of neural stem cells.
The study’s first author, remarked: “We have demonstrated for the first time that the SUMO protein family plays a pivotal role in neural stem cell reactivation and overall brain development. Going a step further, we also showed that when these proteins are absent, normal neuronal development is hampered, with fruit flies developing undersized brains characteristic of microcephaly.”
Delving deeper into the effects of SUMOylation, the researchers determined that it regulates a key protein in another well-known pathway, called Hippo. While the Hippo pathway is known to play a crucial role in cellular processes such as cell proliferation, cell death and organ size, very few regulators of this pathway in the brain are known.
When modified by SUMO, the Hippo pathway’s central protein Warts, which limits cell growth and prevents the reactivation of neural stem cells, becomes less effective. This allows neural stem cells to grow and divide, forming new neurons that contribute to brain function.
The senior author of the study, said: “Given that SUMO proteins and the Hippo pathway are highly conserved in humans, our findings aren’t just relevant for fruit flies. They’re also important for understanding human biology. Disruptions in the SUMOylation process and Hippo pathway are linked to various illnesses in humans, including cancer and neurodegenerative diseases, like Alzheimer’s and Parkinson’s disease. Our new insights into the role of SUMOylation in the brain opens exciting new opportunities for interventions that could lead to targeted therapies that harness the body’s own regenerative powers.”
The team had previously demonstrated that fruit fly neural stem cells are an excellent model for unravelling the mysteries of dormancy, reactivation and neuronal regeneration.
https://www.nature.com/articles/s41467-024-52569-y
https://sciencemission.com/SUMOylation-of-Warts-kinase-promotes-neural-stem-cell-reactivation