Mitochondrial RNA cytosolic leakage drives the SASP
Researchers have uncovered how aging "zombie cells" trigger harmful inflammation that accelerates a severe and increasingly common form of fatty liver disease called metabolic dysfunction-associated steatohepatitis (MASH). As obesity rates rise worldwide, MASH is projected to increase and is already one of the leading causes of liver transplantation.
"Liver scarring and inflammation are hallmarks of MASH. If left untreated, it can progress to liver cancer. This is why it's so important to understand the mechanisms driving the disease so that we can prevent it or develop more effective treatments," says the lead author of the study published in Nature Communications.
As we age, some cells enter senescence — a state in which they stop dividing but continue releasing inflammatory and tissue‑damaging molecules called senescence associated secretory phenotype (SASP). When people are young, the immune system typically eliminates these senescent, or "zombie," cells. With age, however, they can persist and contribute to a range of age‑related health problems and diseases.
While some research focuses on removing these cells, this team investigated how to quiet their harmful signals.
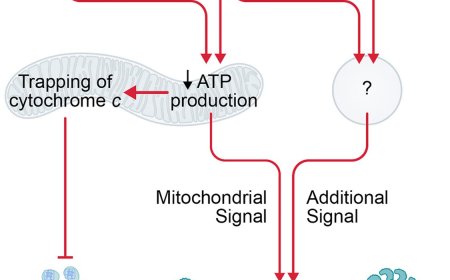
The researchers identified a mechanism by which aged zombie cells drive liver scarring and inflammation. They found that small molecules called mitochondrial RNA, typically found within the cell's energy-producing mitochondria, can leak into the main part of the cell, where they mistakenly activate antiviral sensors called RIG-I and MDA5 — normally triggered when a virus infects a cell. In this case, the danger signal comes from the cell's own mitochondria, prompting a wave of inflammation that can damage nearby healthy tissue.
When the researchers blocked the sensors, inflammation dropped sharply. The study also found that proteins BAX and BAK, which help open pores in the mitochondrial membrane, enable mitochondrial RNA to escape. In a preclinical MASH model, inhibiting BAX and BAK prevented RNA from escaping and was associated with less inflammation and healthier liver tissue.
"With age, we accumulate 'zombie' cells, which can lead to more disease," the senior author of the study. "Our idea is that if we can quiet these cells earlier, we can prevent runaway inflammation and the development of many age‑related conditions, including liver disease. Understanding the mechanisms that drive disease allows us to target and delay those processes — potentially benefiting more than one condition."
https://www.nature.com/articles/s41467-025-66159-z
https://sciencemission.com/Mitochondrial-RNA-cytosolic-leakage