Protein N-terminal modifications
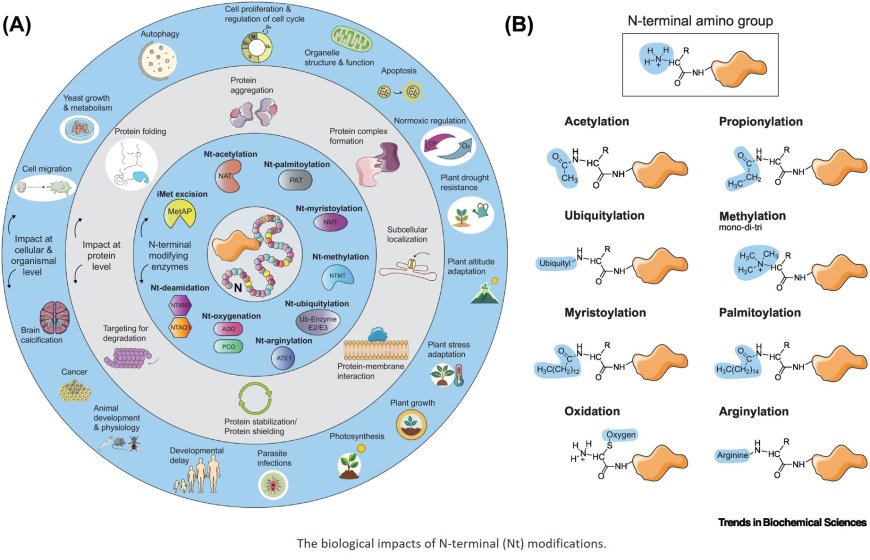
Most eukaryotic proteins interact with at least one of the many N-terminal (Nt)- modifying enzymes, resulting in a large pool of proteins with chemically modified N termini. These Nt modifications include methionine cleavage, acetylation, myristoylation, methylation, ubiquitylation, oxidation, and arginylation, among others.
The enzymes carrying out these Nt modifications can either act co-translationally, when a nascent polypeptide emerges from the ribosome, or post-translationally at a later event.
The Nt-modifying enzymes work in a rather sequence-specific manner to target their substrate proteins. Despite that many of the Nt-modifying enzymes are programmed to target specific Nt amino acid sequences, there are partially overlapping substrate specificities.
Recent work on these Nt modifications implicates them in pathologies, including cancer, neurodegenerative diseases, and infectious diseases, rendering their enzymes candidates for therapeutic targeting.
https://www.cell.com/trends/biochemical-sciences/fulltext/S0968-0004(24)00303-7