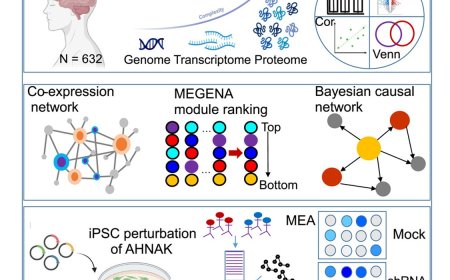
PTP1B inhibition promotes microglial phagocytosis in Alzheimer’s disease
Alzheimer’s disease is often measured in statistics: millions affected worldwide, cases rising sharply, costs climbing into the trillions. For families, the disease is experienced far more intimately. “It’s a slow bereavement,” says the author, whose mother lived with Alzheimer’s. “You lose the person piece by piece.”
There’s a lot of discussion about how the neurodegenerative disorder may be caused by a buildup of “plaque” in the brain. When someone refers to this plaque, they’re talking about amyloid-β (Aβ), a peptide that occurs naturally but can accumulate and come together. This is known to promote Alzheimer’s disease development.
Now, researchers have discovered that inhibiting a protein called protein tyrosine phosphatase 1B improves learning and memory in an Alzheimer’s disease mouse model. Moreover, the researchers show that PTP1B was highly expressed in microglia, and its deficiency promoted a transcriptional shift toward immune activation and phagocytosis. Consistently, PTP1B deletion in microglia enhanced phagocytosis and energy metabolism, supported by increased AKT-mTOR signaling, a pathway essential for meeting the energy demands of activation.
The authors discovered PTP1B in 1988 and have studied this enzyme’s implications for health and disease ever since. In this latest study, the team shows how PTP1B directly interacts with another protein called spleen tyrosine kinase (SYK), which normally regulates microglia (the brain’s immune cells) to clear out debris like excess Aβ. Inhibition of SYK showed that PTP1B modulates microglial activation in a SYK-dependent manner.
“Over the course of the disease, these cells become exhausted and less effective,” says the author. “Our results suggest that PTP1B inhibition can improve microglial function, clearing up Aβ plaques.”
Beyond Aβ, obesity and type 2 diabetes are well recognized risk factors for Alzheimer’s disease and are believed to contribute to its increasing prevalence worldwide. These links provide additional rationale for going after PTP1B in Alzheimer’s disease, as it’s a validated therapeutic target for both metabolic disorders.
Newly approved therapies for Alzheimer’s disease primarily focus on targeting Aβ clearance, yet offer only modest clinical benefits for many patients. “Using PTP1B inhibitors that target multiple aspects of the pathology, including Aβ clearance, might provide an additional impact,” says the author.
The lab is currently working with DepYmed, Inc. to develop PTP1B inhibitors for multiple applications. For Alzheimer’s disease, the author envisions a combination of therapies that pair existing approved drugs along with PTP1B inhibitors. “The goal is to slow Alzheimer’s progression and improve quality of life of the patients,” the author says. With this research establishing PTP1B as a potential therapeutic target for the disease, it may hold the key to doing just that.